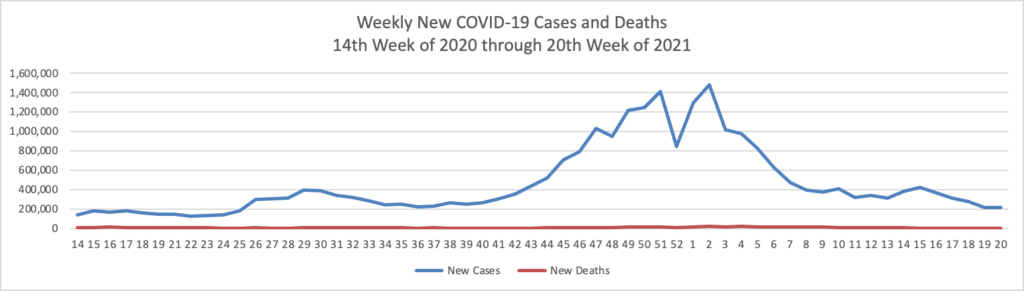
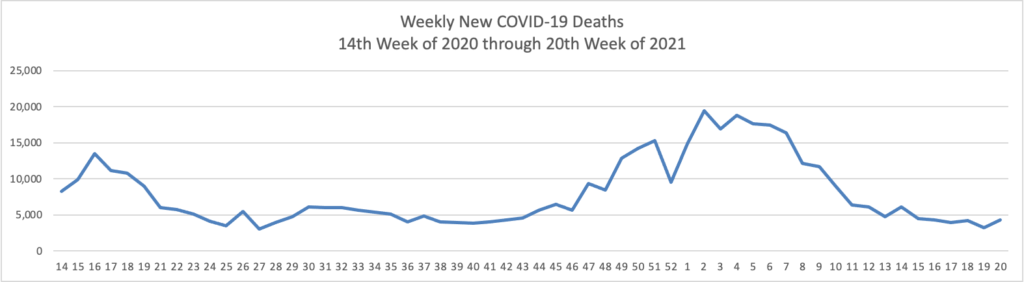
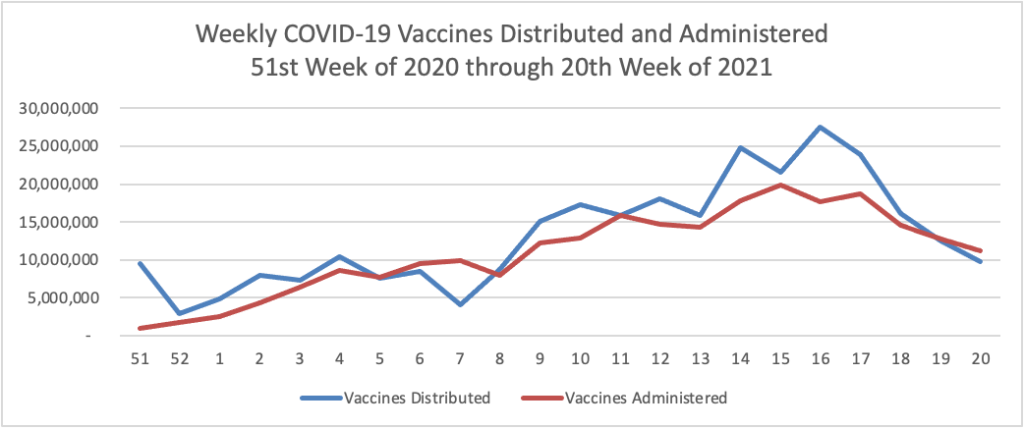
Weekend update

The House of Representatives will be conducting Committee business this week and is not expected to resume floor voting until June 14. The Senate will be conducting Committee business and floor voting this week. Tomorrow the Senate will begin the voting process for confirmed President Biden’s nominee for Centers for Medicare and Medicaid Services Administrator, Chiquita Brooks-LaSure.
The Supreme Court will hold another opinion day tomorrow which may be the occasion for the release of the California v. Texas Affordable Care Act constitutionality decision. Lexology discusses the fallout from the Surpreme Court’s December 2020 opinion in Rutledge v. PCMA narrowing the scope of ERISA preemption with respect to prescription benefit manager law. State legislatures have jumped on the opportunity created by the Rutledge opinion.
In 2021 alone, at least eight states have enacted some sort of PBM reform legislation, including Alabama, Arizona, Arkansas, Mississippi, New York, North Dakota, West Virginia and Wisconsin. PBM reform regulation has passed both the state house and senate in Texas and is on its way to the governor. These bills run the gamut of regulating the PBM industry, from prohibiting PBMs from charging pharmacies fees during and after the claims adjudication process, prohibiting PBMs from reimbursing their own affiliated pharmacies at a higher level than independent pharmacies to banning PBM discrimination against pharmacies participating in the Federal 340B medication discount program. This trend is likely to continue with almost 100 bills introduced across 39 states similarly aimed at regulating the PBM industry
Cost curve up. ERISA decisions like this one impact FEHB preemption because courts have interpreted the two preemption laws as generally analogous in scope.
In other news and opinions:
- Medpage Today offers an op-ed about the importance of primary care. The FEHBlog agrees that “Patients need support for mental and physical health all in one place” and accordingly health plans should encourage the use of primary care.
- Fierce Healthcare reports that “There was a significant increase in pharmacy fraud and abuse under the pandemic, analysts at OptumRx say. The pharmacy benefit manager giant recovered $300 million in fraud, waste and abuse spend in 2020 and documented the largest ever increase in fraudulent claims, which were up 300% compared to 2019. In addition, Optum’s investigative audits led to an increase of 135% in fraud recoveries last year from 2019. The average audit recovery per case was also 70% higher in 2020 than in 2019, Optum found. Optum found the fraudulent behavior concentrated among independent pharmacies and rarely found similar activity among retail chains, [Optum analysts] said. Due to the findings, the PBM axed 112 pharmacies from its network.
- Kaiser Health News informs us that “Colorado health officials so abhor the high costs associated with free-standing emergency rooms they’re offering to pay hospitals to shut the facilities down. The state wants hospitals to convert them to other purposes, such as providing primary care or mental health services. At least 500 free-standing ERs have set up in more than 20 states in the past decade. Colorado has 44, 34 owned by hospitals. The trend began a decade ago with hopes these stand-alone facilities would fill a need for ER care when no hospital was nearby and reduce congestion at hospital ERs. But that rarely happened. Instead, these emergency rooms — not physically connected to hospitals — generally set up in affluent suburban communities, often near hospitals that compete with the free-standing ERs’ owners. And they largely treated patients who did not need emergency care, but still billed them and their insurers at expensive ER rates, several studies have found.” Good luck Colorado as this approach also may reduce surprise billing issues.