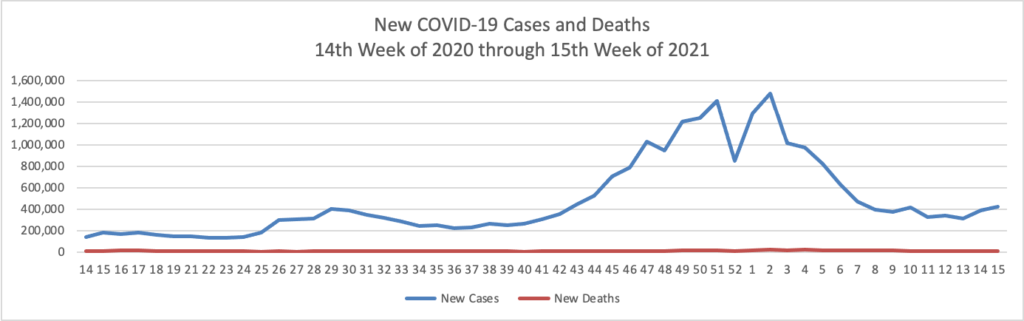
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 15th week of this year (beginning April 2, 2020, and ending April 14, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases greatly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through April 14, 2021):
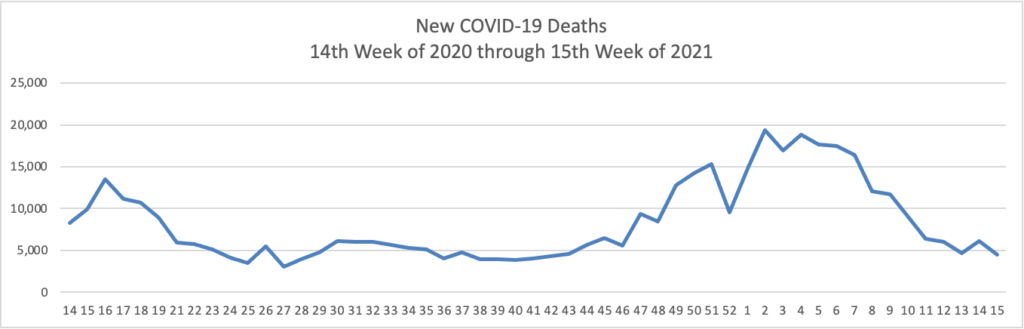
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through April 14, 2021 which also uses Thursday as the first day of the week:
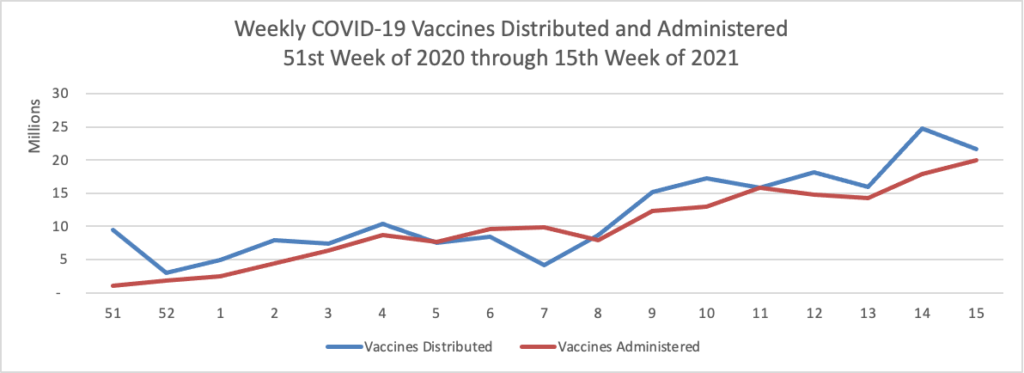
It’s looking reasonably good. According to the CDC, as of today, 49.6% of the U.S. population over age 18, and 80.4% of the U.S. population over age 65, have had at least one dose of the vaccines. 31.1% of the over age 18 population and 64.6% of the over age 65 population are fully vaccinated. Over 200 million doses of the vaccines have been administered in the U.S.
The CDC has announced that “a virtual emergency meeting will be held to discuss Janssen (Johnson & Johnson) COVID-19 vaccine on [Friday] April 23, 2021, 11:00 a.m. to 5:00 p.m. ET” to discuss the ongoing FDA/CDC recommended pause in administration of that vaccine in the U.S. The Wall Street Journal reports that
Johnson & Johnson said Friday there wasn’t enough evidence to establish that the company’s Covid-19 vaccine causes the rare blood-clotting condition that prompted U.S. health officials this week to recommend a pause in its use.
The New England Journal of Medicine published online a letter from three J&J employees involved in vaccine development and epidemiology saying, “At this time, evidence is insufficient to establish a causal relationship between these events” and J&J’s vaccine.
And now for more —
- Beckers ASC Review informs us that Optum “announced plans to add 10,000 physicians in 2021 earlier this year, and Wyatt Decker, CEO of OptumHealth, said Optum is on track to exceed that number [this week]. Optum now has 56,000 affiliated, contracted and employed physicians.” Wow.
- The Food and Drug Administration announced its marketing approval for “Opdivo (nivolumab), in combination with certain types of chemotherapy, for the initial treatment of patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer and esophageal adenocarcinoma. This is the first FDA-approved immunotherapy for the first-line treatment of gastric cancer. ‘Today’s approval is the first treatment in more than a decade to show a survival benefit for patients with advanced or metastatic gastric cancer who are being treated for the first time,’ said Richard Pazdur, M.D., director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research. ‘The FDA is committed to bringing new safe and effective treatment options like Opdivo to patients with advanced cancer.’”
- Fierce Healthcare reports that “Despite the challenges in 2020, physicians’ salaries have rebounded, along with hours working and with only a slight dip in patient volume, according to the Medscape Physician Compensation Report 2021. Based on responses from more than 18,000 U.S. physicians across 29 specialties, the survey—conducted Oct. 6, 2020, to Feb. 11, 2021—found that average salaries for primary care physicians held steady at $242,000 from $243,000 the previous year. Similarly, specialists’ average salaries dropped $2,000 to $344,000.”
- Fierce Healthcare also reports
Ride-sharing company Lyft is letting patients schedule nonemergency medical transport (NEMT) on health organization’s dime with the launch of Lyft Pass for Healthcare. The latest healthcare offering falls in line with the initial Lyft Pass service launched in July 2020, which allows business organizations to monitor and cover the cost of employees’ transportation. Now, the company is extending those capabilities to healthcare organizations—commercial health plans as well as Medicare or Medicaid—and their members.
Through the app, users who need a ride to their medical appointments, vaccinations, prescription pickups or other destinations request a ride. This process is similar to ordering a pickup as a consumer, except that patients will need to select an in-app branded Lyft Pass provided via phone number, access code or a direct link. Healthcare organizations sponsoring the pass, meanwhile, are able to customize the program’s budgets, approved locations and scheduling windows. The organizations are able to monitor usage and manage spend while allowing members to be more autonomous with their NEMT scheduling.
