Monday Roundup

From the federal policy front, Fierce Health tells us about four healthcare policy changes beyond government negotiation of a subset of Medicare-covered drugs and extension of ACA subsidies found in the budget reconciliation bill that Congress approved last week. The President is scheduled to sign the bill into law tomorrow.
Here are four other health policy changes to look for in the bill:
- First, expands eligibility for the full amount of low-income Part D subsidies from 135% of the federal poverty line to 150% of the FPL.
- Gets rid of the cost-sharing for adult vaccines for Medicare Part D. The FEHBlog has personal experience with this one. In 2020 he received the new Shingrix vaccine under Medicare Part D. The vaccine was eligible for no cost sharing when administered in-network under the Affordable Care Act. However, under Part D, the FEHBlog was charged $200 for each of the two doses. This big bowl of wrong will be remedied in 2023.
- Delays the controversial Part D rebate rule, again. “The Trump-era rule would eliminate the safe harbor for Part D rebates, leaving them open to prosecution under federal anti-kickback laws. The rule passed at the tail end of Trump’s term but has never gone into effect. The law would delay the rule from going into effect again into 2032.”
- Limits the premium growth on Medicare Part D to no more than six percent a year from 2024 through 2029. “The cap on premium growth is intended to mitigate the impact of other changes to Part D, said Ryan Urgo, managing director of the policy practice at consulting firm Avalere Health. The legislation includes a $2,000 out-of-pocket cost cap on Part D drugs, spread out in installments for the beneficiary over a calendar year. Part D plans will also have to pick up more of the costs for spending in the catastrophic coverage phase, which a beneficiary reaches when their drug costs reach a certain level.”
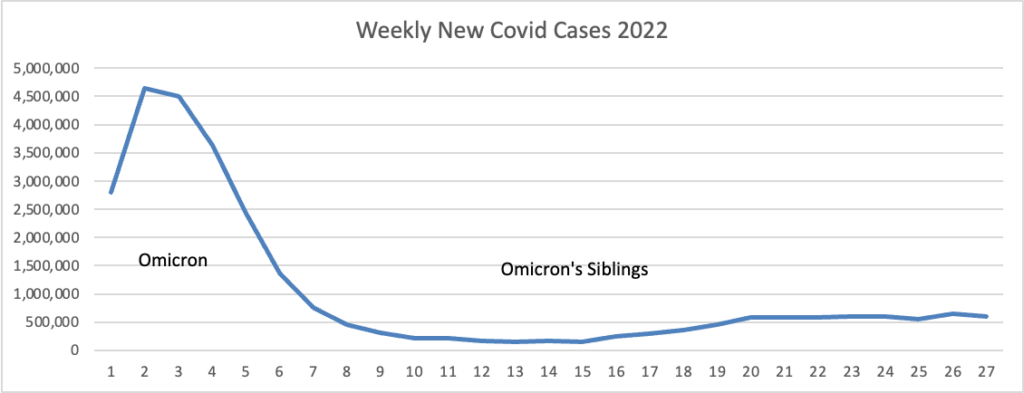
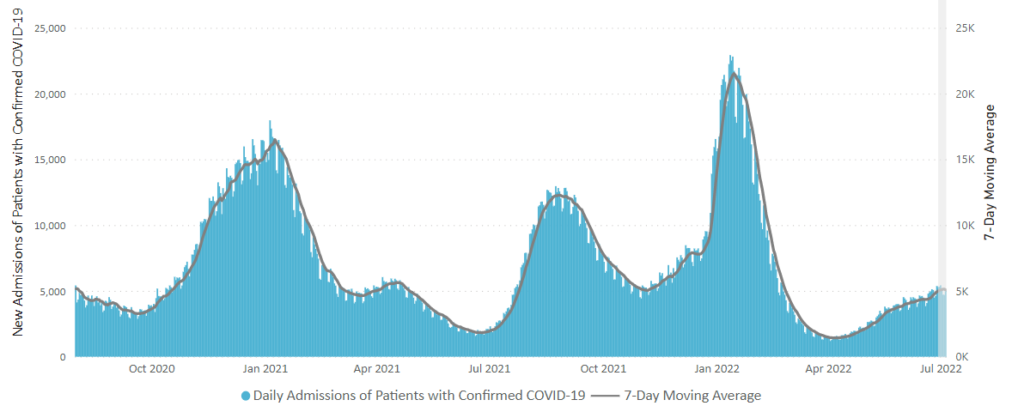
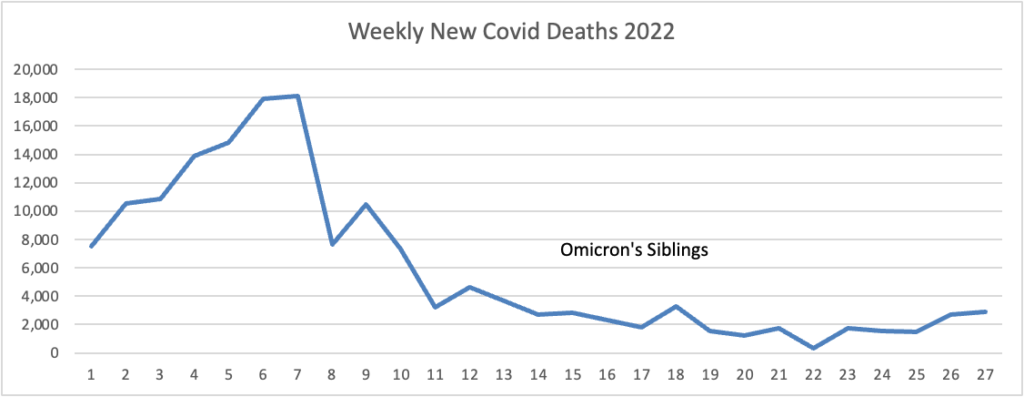
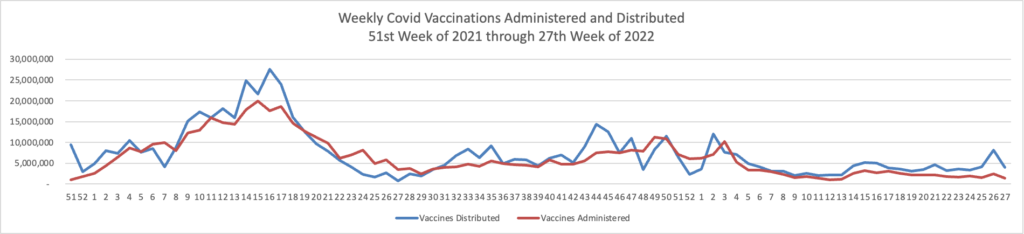
From the Omicron and siblings front, BioPharma Dive informs us
British drug regulators on Monday approved a two-pronged vaccine from Moderna that’s designed to fight both the original version of the coronavirus and the omicron variant.
The decision makes the U.K. the first country to clear a COVID-19 booster that’s been specifically tailored to a newer form of the virus. While the original shots, like Moderna’s Spikevax, remain strongly effective at preventing hospitalization and death, they’ve had a harder time warding off infections from omicron and its subvariants. * * *
Speaking to a [U.S.] advisory committee in June, executives from Moderna and Pfizer said dual-acting boosters with a BA.4 or BA.5 could be available in large volumes in October and November, although they would first need to undergo regulatory review before they could be administered.
Separately, Novavax on Monday asked the FDA to authorize its COVID-19 vaccine as a booster shot in people 18 and older. The shot is only available as a primary series to those who haven’t yet been vaccinated.
The Centers for Disease Control released updated guidance on what to do if exposed to Covid.
The Wall Street Journal reports on assessing Covid-19 risks after easing CDC guidelines, which began last week. The headline notes, “More decision-making is shifting to individuals, and here is what doctors recommend.”
From the monkeypox front, the Journal discusses “What to Know About Symptoms, Vaccines and How It Spreads,” and Beckers Hospital Review offers five monkeypox updates, including a complete explanation of how the World Health Organization is going about changing the disease’s name.
From the mental healthcare front, about ten years ago, the FEHB Program under OPM’s leadership was the first large employer-sponsored program to cover a form of autism therapy called “applied behavior analysis” (ABA). A STAT News investigation discloses
ABA has long been viewed as the gold standard for kids with autism, so much so that every state mandates insurance coverage. For some families, it is the only option that insurance will cover at all.
But like other pockets of the health care industry, this one has been transformed over the past decade by a flood of investments from private equity firms, drawn by the promise of insurance reimbursement and the rising rate of autism in children across the U.S., now estimated at 1 in 44 kids.
Families and clinicians who once believed fully in the promise of ABA say the financial investors’ fixation on profit has degraded the quality of services kids receive, turning it into the equivalent of fast food therapy. They’ve grown disillusioned with the industry, they told STAT.
The HHS Inspector General is auditing Medicaid claims for ABA. The final audit report is expected later this year.
From the Rx coverage front, the Drug Channel blog discloses
Here’s a summer surprise for fans of the 340B Drug Pricing Program: Drug Channels has just obtained the 2021 figures from the Health Resources and Services Administration (HRSA)! Even better, my Freedom of Information Act (FOIA) request was able to pry out detailed purchases by covered entity type.
The data tell a familiar story. For 2021, discounted purchases under the 340B program reached a record $43.9 billion—an astonishing $5.9 billion (+15.6%) higher than its 2020 counterpart. Hospitals accounted for 87% of these skyrocketing 340B purchases.
What’s more, the difference between list prices and discounted 340B purchases also grew, to $49.7 billion (+$7.0 billion). This figure approximates the money collected by 340B covered entities.
340B advocates have been screaming that “drug companies are cutting 340B,” but the data tell a very different story. Only in the U.S. healthcare system can billions more in payments and spreads be considered a cut.
From the U.S. healthcare business front, Beckers Payer Issues provides an interview with “Kyu Rhee, MD, a senior vice president at CVS Health and chief medical officer at Aetna. He sat down with Becker’s to discuss ongoing trends across the healthcare industry and how he is working to create a “values-based” care system through opportunities offered by a global pandemic.”