Friday Stats and More
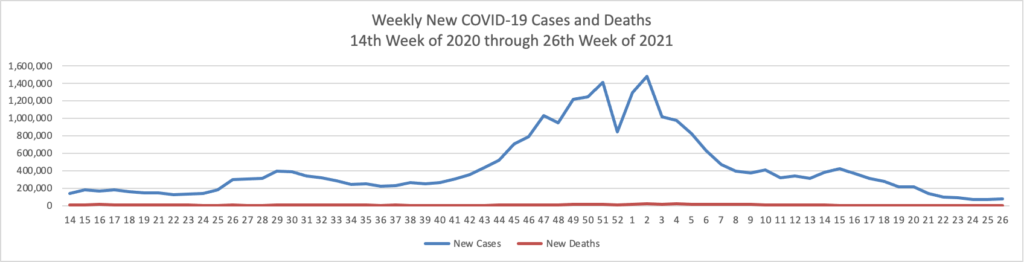
Based on the Centers for Disease Control’s COVID Data Tracker and using Thursday as the first day of the week, here is the FEHBlog chart of weekly new COVID cases for the end of June 2021 through the first week of 2022:

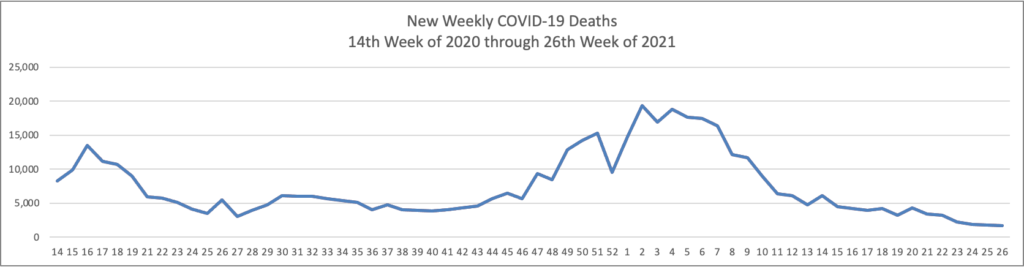
Omicron has produced a staggering number of new COVID cases while the number of weekly new COVID deaths has remained with a stable range for several months.

The Wall Street Journal offers an lab test based explanation for Omicron’s mild nature:
The threat posed by the Omicron variant has now come into sharper focus, with recent clinical data and laboratory studies lending support to early reports suggesting that it is milder but more transmissible than other variants of the new coronavirus.
“It spreads very, very fast, but it doesn’t appear to have the virulence or machismo to really pack as much of a wallop as the Alpha or Delta variants,” James Musser, chairman of Houston Methodist Hospital’s pathology and genomic medicine department and the leader of a new study of Omicron infections, said of the variant.
Recent laboratory studies suggest that Omicron’s lower virulence may reflect its apparent tendency to thrive in cells in the upper respiratory tract rather than in the lungs, where Covid-19 infections can cause potentially fatal breathing problems.
That’s a reassuring tidbit.
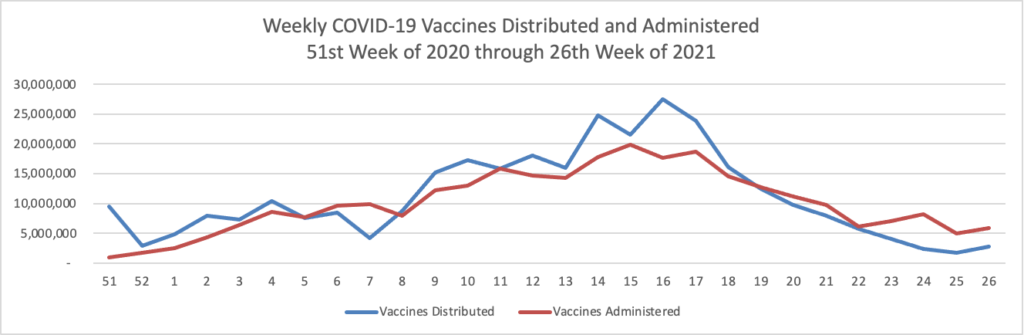
Here’s the FEHBlog’s weekly chart of COVID vaccinations from the 51st week of 2020 through the 1st week of 2022:

Throughout the holiday period the number of administered vaccinations averaged six million which is impressive. Nearly two thirds of Americans aged five years and older are fully vaccinated and half of Americans aged 50 and older also have received a booster.
Here are a link to the CDC’s interpretation of its COVID statistics and a link to the CDC’s Fluview.
From the Supreme Court front, the Wall Street Journal reports that
A majority of Supreme Court justices expressed skepticism Friday of the Biden administration’s Covid-19 vaccine-or-testing plan for large employers but somewhat less concern about a vaccination mandate for healthcare workers, in a special [four hour long] session that examined the scope of the federal government’s powers during a fast-moving pandemic.
The Court focused its attention whether the issuing agency has necessary authority to issue its broad mandate. The Court should issue a decision within a month. The Wall Street Journal adds
The Supreme Court took up the vaccine lawsuits with alacrity, acting shortly before Christmas to add a rare Friday argument to its docket, ahead of the year’s first scheduled cases. Some parts of the OSHA rules begin to take effect next week, though the agency is waiting until next month to enforce Covid-19 testing requirements. Several justices signaled the court would seek to rule quickly—and potentially could put the requirements for private employers on hold temporarily for at least a few days to give themselves time to digest the case.
From the Medicare front, the Centers for Medicare and Medicaid Services released a proposed rule with 2023 updates to the Medicare Advantage and Medicare Part D programs. Here are links to the CMS fact sheet and a Fierce Healthcare article on this development. Of note, Fierce Healthcare tells us that
The proposal takes a major aim at price concessions that Part D plans extract from drug makers, but does not affect rebates negotiated between drug makers and insurers. Under the concessions, the plan pays less money to a pharmacy if it doesn’t meet several metrics. CMS is concerned, however, that the end-user doesn’t know about the arrangement and the lower prices are not passed on at the point-of-sale.
The proposed rule also said that the negotiated prices “typically do not reflect any performance-based pharmacy price concessions that lower the price a sponsor ultimately pays for the drug.”
The proposed rule wants to require all Part D plans to apply the concessions at the pharmacy counter.
Fierce Healthcare also calls attention to the November 2020 Kaufmann Hall report on hospital service utilization.
Hospital volumes softened in November overall as operating margins remain depressed, signaling that once again consumers could be delaying or avoiding care due to the pandemic, a new report from consulting firm Kaufman Hall found.
The firm released Tuesday its latest hospital flash report detailing revenues and volumes for November before the omicron-fueled surge of COVID-19 took hold. The report found hospitals are still facing major pressures from rising expenses and labor shortages. * * *
Kaufman found that hospital volumes softened in November, with discharges dropping nearly 5% and adjusted discharges by 3.9% compared to the month before. Discharges were also down 6.1% compared with pre-pandemic levels.
Meanwhile, the average length of stay at hospitals increased by 0.8% compared to October and 8.6% compared with November 2019.
The report estimates consumers could be postponing non-COVID-19 care.
“The potential impact of the omicron variant in future months may influence this trend further,” Kaufman’s analysis said.