Friday Stats and More
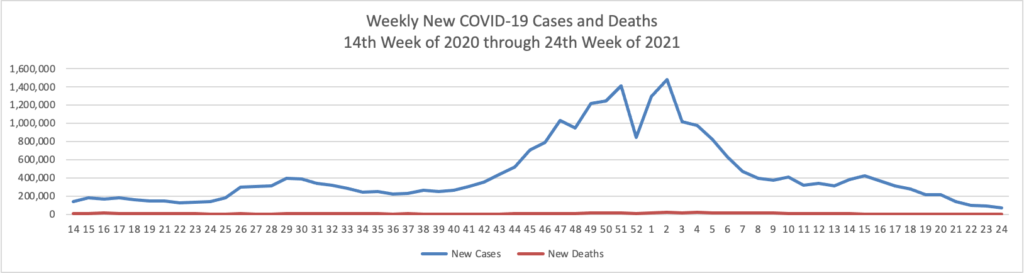
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 24th week of this year (beginning April 2, 2020, and ending June 16, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases materially exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through June 16, 2021):
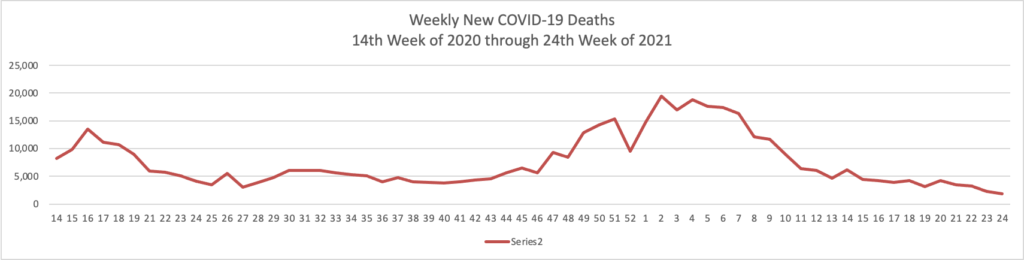
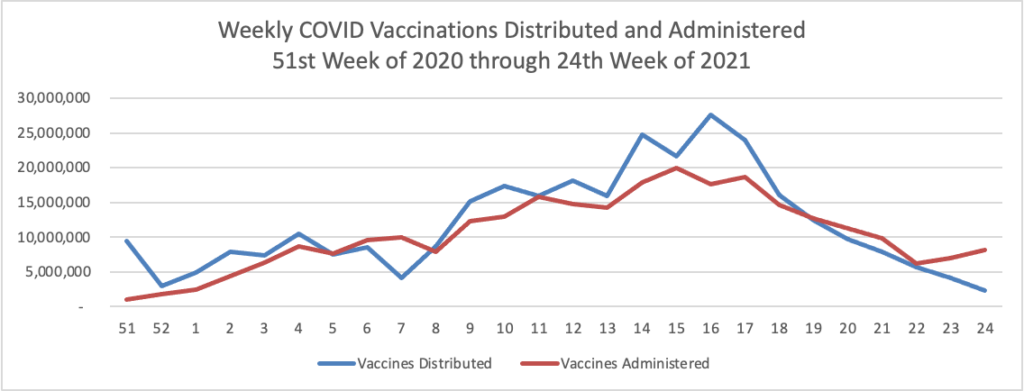
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through June 16, 2021 (six months) which also uses Thursday as the first day of the week:
Bloomberg reports that
President Joe Biden urged unvaccinated Americans to get inoculated from coronavirus, warning that the highly transmissible delta variant of the virus could cause more deaths. “Even while we are making incredible progress [as reflected above], it remains a serious and deadly threat,” Biden said Friday during a White House event to celebrate 300 million doses of vaccine administered during the first 150 days of his administration.“The data is clear: If you are unvaccinated, you’re at risk of getting seriously ill, or dying, or spreading it.” A large swath of Americans — particularly in the politically conservative South — have declined shots despite warnings from health authorities that the virus remains a threat.”
in other COVID-19 news
- Medscape informs us that “While the investigation into cases of myocarditis possibly associated with COVID vaccines proceeds, the American Heart Association (AHA)/American Stroke Association (ASA) continue to urge everyone who is eligible for the vaccine to get it without delay. ‘We remain confident that the benefits of vaccination far exceed the very unusual risks,’ the leadership of the AHA/ASA said in a statement issued June 12. ‘The risks of COVID-19 infection include its potentially fatal consequences and the potential long-term health effects that are still revealing themselves, including lingering consequences affecting the heart, brain, vascular system, and other organs after infection,’ they point out. Late last week, the Centers for Disease Control and Prevention (CDC) alerted healthcare providers that the COVID-19 Vaccine Safety Technical Work Group (VaST) of the Advisory Committee on Immunization Practices (ACIP) will meet June 18 to review cases of myocarditis reported in adolescents and young adults after they received a COVID-19 vaccine manufactured by Pfizer–BioNTech or Moderna.
- MedPage Today reports that “During the American Medical Association (AMA) House of Delegates annual meeting, members debated whether natural immunity or previous infection with SARS-CoV-2 was sufficient for the merit of immunity credentials.” The House of Delegate voted against treating natural immunity as equivalent to vaccination. “Multiple delegates pointed out that using natural immunity in lieu of vaccination would pit the AMA’s recommendations against those of the CDC.” Nevertheless as the Mayo Clinic points out, natural immunity helps us achieve some level of herd immunity. It needs to be considered with vaccinations for that purpose.
If you want more details on yesterday’s Affordable Care Act decision from the U.S. Supreme Court, check out Prof. Katie Keith’s post in the Health Affairs blog.
Beckers Hospital News lists seven Fortune 500 health insurers by membership. Health Payer Intelligence discusses the latest sustainability report from the company that tops this list, UnitedHealth Group.
On this Juneteenth holiday, Fierce Health tells us about how “healthcare executives call on President Biden to take ‘innovative, bold’ steps to tackle health equity using AI, big data.”
“It’s not a lack of data—we have so much data in this country now, in our healthcare systems and our EHRs and our patient registration systems,” Cole said. “The data is there but the analytics capability of that—what to do with that data—is something that we’re continuing to work on every day.”
Cole and John Lumpkin, M.D., president of the Blue Cross and Blue Shield of North Carolina Foundation, said both of their organizations have been reworking their collection and organization of race, ethnicity, gender identity and other related data tied to health inequity—a change that “should be a very simple thing to do” but requires an internal data system overhaul, Lumpkin said.
Still, executives said the finish line of those efforts is worthwhile. Incorporating SDOH data can yield substantial health and costs benefits at the individual and population levels, they said.
The Wall Street Journal reports on the rising fortunes of the country’s major pharmacy chains, CVS Health and Walgreens.
In health system merger news —
- Healthcare Dive informs us that “Beaumont Health and Spectrum Health are looking to merge in a deal that would result in Michigan’s biggest health system with 22 hospitals and 64,000 employees across the state, with combined annual revenue of almost $13 billion. Executives of the two systems announced Thursday they have signed a letter of intent to explore creating a joint health system. The deal would include Spectrum’s Michigan-based health insurance plan, Priority Health, which has 1.2 million customers.”
- Fierce Healthcare reports that “Southern health systems Ochsner Health and Rush Health Systems have announced plans for a merger they expect to be completed about halfway through 2022. The deal follows a 2019 strategic partnership between the two nonprofit providers and comes hot on the heels of Louisiana-based Ochsner’s merger with Lafayette General Health, which closed in October 2020 and grew the system to 35 hospitals. Rush, which has seven hospitals and more than 30 clinics in eastern Mississippi and western Alabama, will be rebranded as Ochsner Rush Health should the merger receive regulatory approval.”
- Healthcare Dive also tells us that “Tenet, a major U.S. health system, has agreed to sell five hospitals in the Miami-Dade area for $1.1 billion to Steward Health Care System, a physician-owned hospital operator and health network. The deal also includes the hospitals’ associated physician practices. Dallas-based Steward has agreed to continue using Tenet’s revenue cycle management firm, Conifer Health Solutions, following the completion of the deal, which is expected to close in the third quarter. Further underscoring Tenet’s strategic focus, the sale will not include Tenet’s ambulatory surgery centers in Florida. Tenet will hold onto those assets as its ambulatory business becomes a bigger focus for the legacy hospital operator.”








