Monday Roundup

From Capitol Hill, Federal News Network discusses the federal employee pay raise angles presented by the House financial service and general appropriations bill which cleared the House Appropriations Committee last Friday. Federal News Network indicates that the bill leaves the door open for the Senate to also accept the President’s proposed 2023 4.6% pay raise for federal employees and the military.
From the Dobbs case front, Govexec.com reports
President Joe Biden announced two actions immediately after the ruling: one directing the Department of Health and Human Services to safeguard access to contraception and medication abortion, and another protecting travel for medical appointments.
To those ends,
- Govexec tells us that OPM today confirmed that its policy allowing federal employees to apply sick time to travel out of state remains in effect after the high court struck down Roe v. Wade, and
- The Department of Health and Human Services (HHS) announced that a meeting was held today between Affordable Care Act regulators, including the HHS and Labor Department Secretaries, and health plan executives to emphasize the importance of full compliance with the ACA’s contraceptive coverage with no cost-sharing mandate when delivered in-network. The ACA regulators also issued a letter to health plans making the same point.
The FEHBlog ran across this NPR Shots article which explains that the Plan B or morning-after pill is considered a contraceptive and not an abortion drug. The Wall Street Journal informs us
Some of the nation’s biggest retailers are rationing over-the-counter emergency contraceptive pills as demand spikes following the Supreme Court ruling overturning a constitutional right to abortion.CVS Health Corp., Walmart Inc., and Rite Aid Corp. were limiting purchases of the pills, which were in short supply or out of stock Monday morning on major retailer websites. CVS and Rite Aid were limiting purchases to three. Walmart had some pills available without limits, but only in cases where they wouldn’t ship until next month. Pills available this week were limited to four or six.
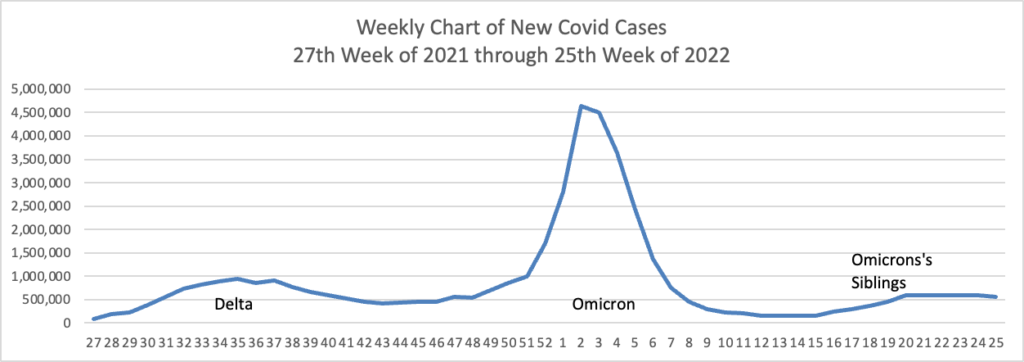
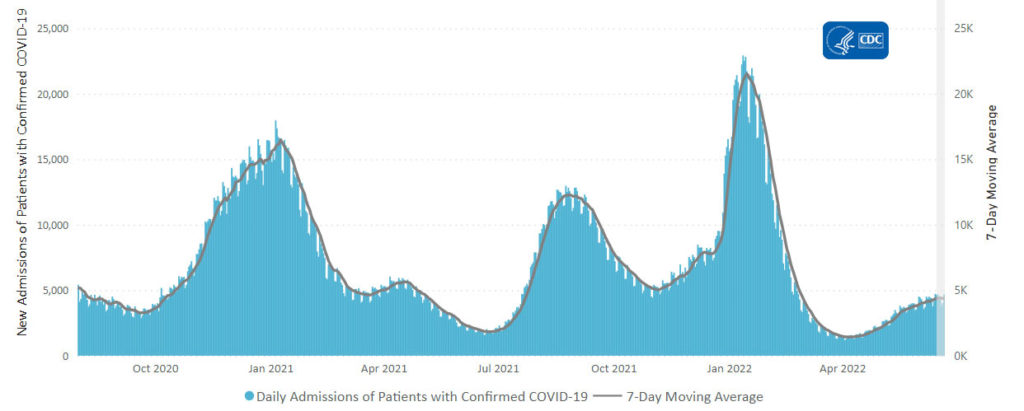
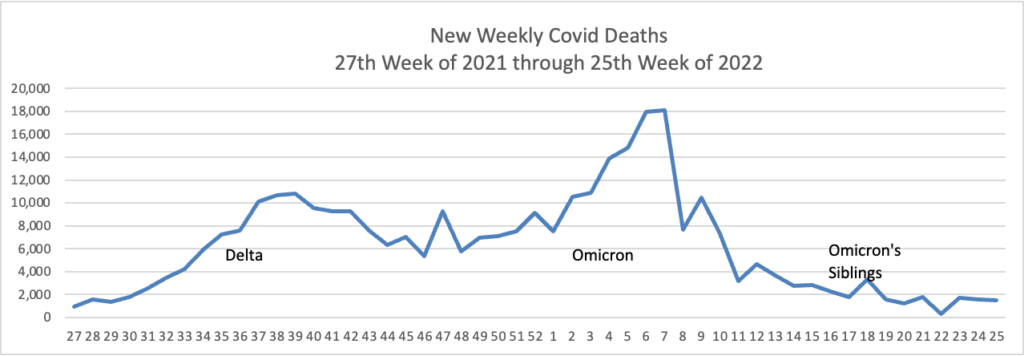
From the Omicron and siblings and monkeypox front
- Govexec reports on a U.S. Court of Appeals for the Fifth Circuit decision order rehearing a federal employee vaccine mandate case which upheld the mandate on lack of plaintiffs’ standing to challenge the mandate. The mandate nevertheless has remained on hold while the case winds it way through the appellate court.
- USA Today reports on when and how to access the monkeypox vaccine.
From the Medicare front, HHS announced
a new model aimed at improving cancer care for Medicare patients and lowering health care costs. CMS’ Center for Medicare and Medicaid Innovation (Innovation Center) designed the Enhancing Oncology Model (EOM) to test how to improve health care providers’ ability to deliver care centered around patients, consider patients’ unique needs, and deliver cancer care in a way that will generate the best possible patient outcomes. The model will focus on supporting and learning from cancer patients, caregivers, and cancer survivors, while addressing inequities and providing patients with treatments that address their unique needs.
From the reports and studies department —
- The next issue of Health Affairs offers a bevy of articles on Type 2 diabetes and pre-diabetes which are available at this link.
- The Congressional Budget Office has made available examples of the work performed by its Health Analysis Division.
- HealthDay reports “More than 18 million Americans have now survived cancer, a new report shows. The American Cancer Society (ACS) and the U.S. National Cancer Institute collaborated on the report to estimate cancer prevalence and help public health officials better serve survivors.”
- mHealth Intelligence calls our attention to a telehealth-oriented Healthcare Experience Report: 2022 released by Zocdoc. The FEHBlog was pleased to read “Mental health continues to hold a place of dominance in telehealth. In May of 2020, 2021, and 2022, the percentage of mental health visits that occurred virtually was 74 percent, 85 percent, and 87 percent, respectively.” Hub and spoke telehealth, e.g, Teladoc, brings mental health care in-network thereby lowering benefit costs while improving access to care.