Midweek update

From the Delta variant front, the Safer Federal Workforce group issued updated guidance for federal employees receiving a COVID-19 vaccine. In short, federal agencies should
- Allow employees to take up to 4 hours of administrative leave to get any COVID-19 dose.
- Allow employees to take up to 2 days of administrative leave for adverse reactions to any COVID-19 vaccination dose.
Federal News Network informs us that
Nearly one month after the Biden administration first announced plans to adopt a vaccine and testing policy for federal employees and contractors, managers — presumably the ones charged with implementing and enforcing the new program on the ground level — say they’re still looking for answers about how it’ll work. * * *
Guidance has been “minimal” and the planning to date has been “stressful” for managers and supervisors, said Craig Carter, national president of the Federal Managers Association.
Professional associations don’t have exact data and they’re relying on anecdotal conversations with their members about the vaccine. But considering a little more than half of all Americans are fully inoculated against COVID-19 — and the federal workforce is in many ways a representative subset of the American public — they assume roughly 50% of the nation’s 2.1 million federal employees are unvaccinated.
That means agencies may potentially need to test about 1 million federal workers once or twice a week, the associations said.
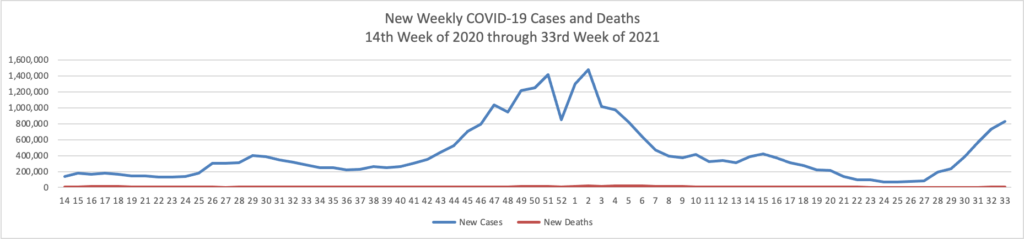
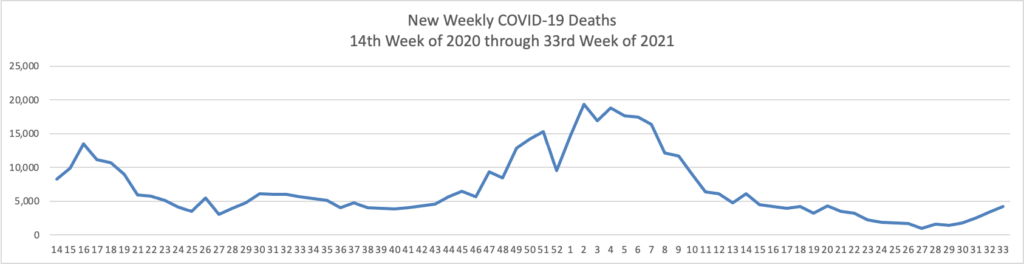
Few things drive the FEHBlog crazier than the use of the full U.S. population as a COVID-19 vaccination benchmark because people under age 12 cannot be vaccinated at this point. 62.7% of Americans over 18 and 81.3% of Americans over 65 are fully vaccinated according to the CDC website. The FEHBlog therefore expects that at least two thirds of federal employees are fully vaccinated. The percentage should skew higher because as the FEHBlog has noted about 20% of the federl workforce is under a COVID vaccination mandate as opposed to attestation. Nevertheless, testing about 600,000 federal employees once or twice a week is no picnic for federal managers.
The Wall Street Journal reports that
- “U.S. Covid-19 hospitalizations have surpassed 100,000 for the first time since January, nearly doubling since the start of August. While the figure remains below the country’s winter peak, hospitals in some parts of the U.S. are straining under the load, and officials in states including Georgia, Kentucky, Tennessee and Idaho have requested extra personnel and resources.
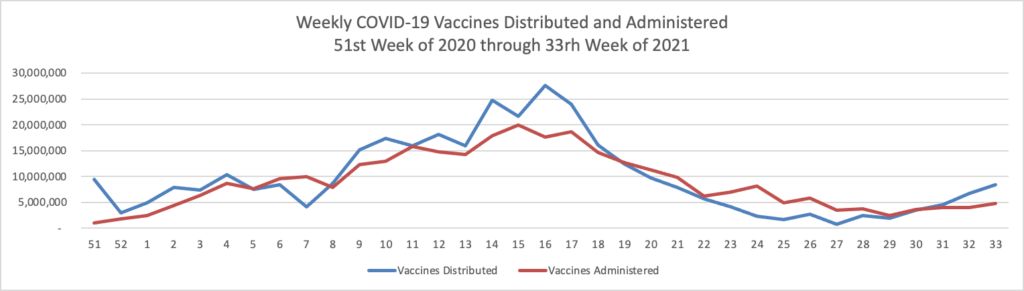
- “Federal regulators are likely to approve a Covid-19 booster shot for vaccinated adults starting at least six months after the previous dose rather than the eight-month gap they previously announced, a person familiar with the plans said, as the Biden administration steps up preparations for delivering boosters to the public.”
The Journal also offers its perspective on the lay of the land for COVID-19 tests.
Employee Benefits News tells us that
According to the most recent Mental Health Index by Total Brain and the National Alliance of Healthcare Purchaser Coalitions, feelings of anxiety increased 94% from June to July, and incidences of PTSD have spiked 83% over the past six months.
While employees of all ages are struggling to maintain good mental health, workers aged 40-59 saw the highest increases in stress, anxiety and feelings of negativity, compared to July’s data. These workers cited return to work and back to school plans as the main drivers of their fears.
The average age of a federal employee is close to 50 years old.
From the federal employee benefits front, Reg Jones discusses the pluses and minuses of deferred annuities.
It’s a fact of life that many people work for a number of years in a job and, for one reason or another, leave before they are eligible to retire. What’s different for those who work for the federal government is that during their working time there, deductions have been taken from their pay toward a civil service annuity.
While many who resign from the government ask for a refund of those contributions, some do not (often because they were not even aware that they could). Those who leave their contributions in the fund – especially those who weren’t even aware that they could get a refund – are the people I want to talk to today, as well as any of you who are thinking about resigning from the government before retirement eligibility.
Here’s why: If you leave your contributions in the retirement fund, you will be entitled to a deferred annuity if you meet some fairly minimal requirements [as explained in the article].
In healthcare business news
- Fierce Healthcare reports that “Hospitals and health systems’ economic recovery hit the brakes in July with mounting COVID-19 admissions, escalating expenses and early evidence that consumers are again postponing elective and outpatient care. Per the latest monthly report from Kaufman Hall, countrywide margins and volumes remained below pre-pandemic numbers but dipped most severely in the South and Midwest, where COVID-19 has had the greatest impact. The firm said it expects these trends to continue in the coming months.”
- Healthcare Dive tells us that “GuideWell, the parent company of Blue Cross and Blue Shield of Florida, is set to acquire Triple S Management, the Blue Cross Blue Shield Plan and largest insurance carrier in Puerto Rico. The $900 million cash deal will add another company to GuideWell’s portfolio of health-focused subsidiaries. After the deal is complete, Triple S will become a wholly owned subsidiary of GuideWell and will continue to operate under the same brand name and management team, the two companies said Tuesday. The deal is expected to close in the first half of next year and is subject to regulatory approvals.”
- Fierce Healthcare also reports that “Carbon Health, a primary care provider combining brick-and-mortar clinics with virtual services, bought two separate clinic chains to expand its national primary care footprint. The company bought Southern Arizona Urgent Care’s nine clinics in Tucson, Arizona, and Med7 Urgent Care’s four clinics in Sacramento, California, bringing its total to 83 clinics across 12 states. This acquisition underscores the company’s goal of becoming the largest national healthcare provider, fueled by its recent $350 million funding news.