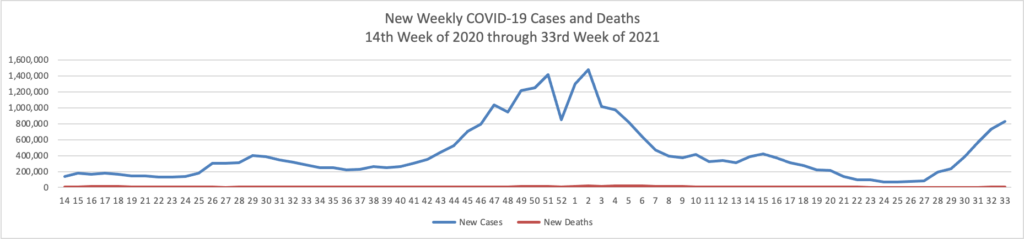
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 33rd week of this year (beginning April 2, 2020, and ending August 18, 2021); using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases significantly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through August 18, 2021):
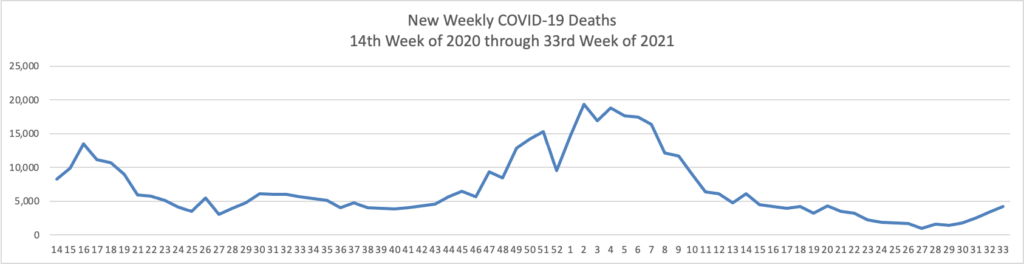
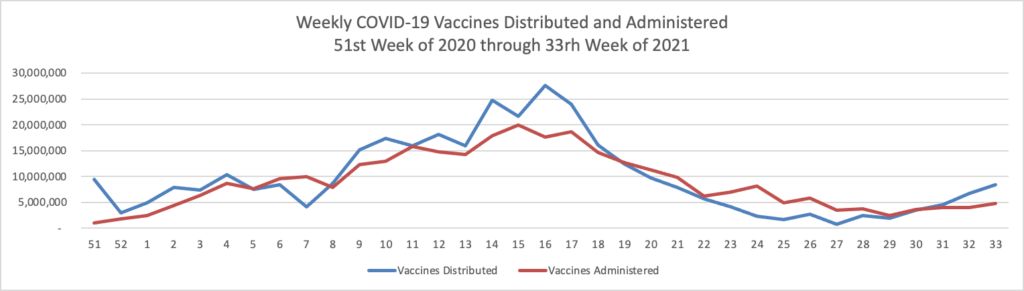
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through August 18, 2021, which also uses Thursday as the first day of the week:
Here’s a link to the CDC’s weekly interpretation of its COVID-19 statistics.
Politico reports that “More than one million Americans received a dose of Covid-19 vaccine on Thursday[August 19] , a benchmark the nation has not met in nearly seven weeks amid a resurgence of the coronavirus pandemic.”
HealthDay informs us that
Antibodies generated by COVID-19 vaccines are effective against the Delta variant and other coronavirus variants of concern, new research shows.
The findings may help explain why most vaccinated people have avoided the surge of Delta variant cases sweeping across the United States.
“In face of vaccination, Delta is relatively a wimpy virus,” said study co-author Ali Ellebedy, an associate professor of pathology and immunology at Washington University School of Medicine in St. Louis.
Good to hear.
The Wall Street Journal tells us that
The Food and Drug Administration is expected next week to grant full approval of the Covid-19 vaccine from Pfizer Inc. and partner BioNTech SE, according to people familiar with the planning, an action that could spur more vaccination requirements by employers and encourage more people who are hesitant to get vaccinated. * * *
Once fully approved, the vaccine would be eligible for off-label prescriptions, such as booster doses, according to the FDA. However, analysts said, the critical element for broad boosting is a recommendation from the Advisory Committee on Immunization Practices to the FDA, as physicians often follow ACIP recommendations.
With full approval, Pfizer would likely be permitted to market the vaccine to doctors, providers and the general public as it does with other approved products. The FDA is permitted to restrict such communications with emergency authorization.
The Journal adds that
Of the three authorized vaccines in the U.S., only Pfizer has submitted all the required information to the FDA, according to the companies, and analysts expect it to be the first receive clearance.
Moderna Inc., whose authorized two-dose shot uses similar mRNA technology as the Pfizer-BioNTech, has said it is still completing rolling data submissions.Johnson & Johnson, whose shot was authorized in February, has said it plans to file for full approval later this year.
Fierce Healthcare reports that Janet Woodcock will not be nominated for a promotion from acting to permanent Food and Drug Commissioner due in large part to Aduhelm fallout.
From the regulatory front
- After issuing a minimalist Affordable Care Act (“ACA”) FAQ 48 earlier this week, the ACA regulatory departments issued a blockbuster ACA FAQ 49 about payer transparency rule implementation and enforcement delays and answering many No Surprises Act (“NSA”) implementation issues left hanging by the first NSA interim final rule (“IFC”) released July 1.
- OMB’s Office of Information and Regulatory Affairs (“OIRA”) has scheduled more meetings on the second No Surprises Act IFC which concerns the independent dispute resolution process. Those listening sessions now run late into next week. That means that the IFC won’t be released before the week of August 30 but that still would be about a month before the statutory deadline.
- The American College of Emergency Physicians and Blue Cross each met with OIRA this past week. Here are links to their supporting letters. BCBSA OIRA IDR 8.17.21.pdf and ACEP EDPMA Pre-IDR Rulemaking Letter (8.10.21).pdf Common sense as expressed in the Blue Cross letter must prevail if the parties want to avoid having the second IFC also create major system changes.
- The Society for Human Resource Management informs us that “covered employers [such as FEHB plan carriers] now have until Oct. 25 to file their 2019 and 2020 EEO-1 reports, according to a recent announcement from the U.S. Equal Employment Opportunity Commission (EEOC). Although the reporting deadline has been delayed several times during the COVID-19 pandemic, the agency said it will not authorize any more extensions.”
