Midweek Update

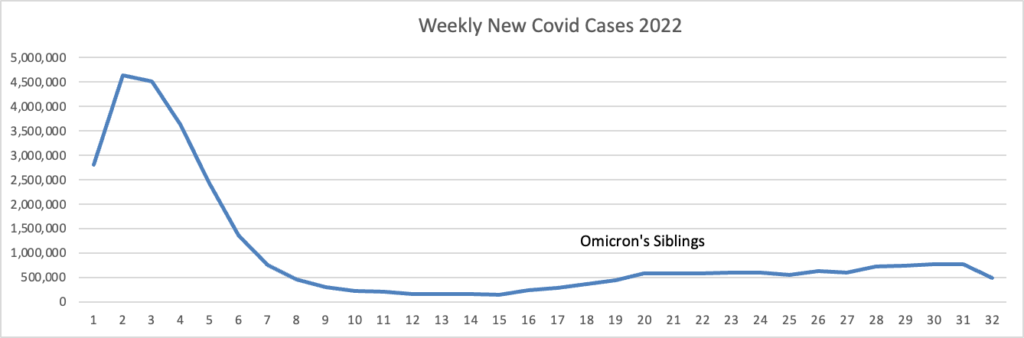
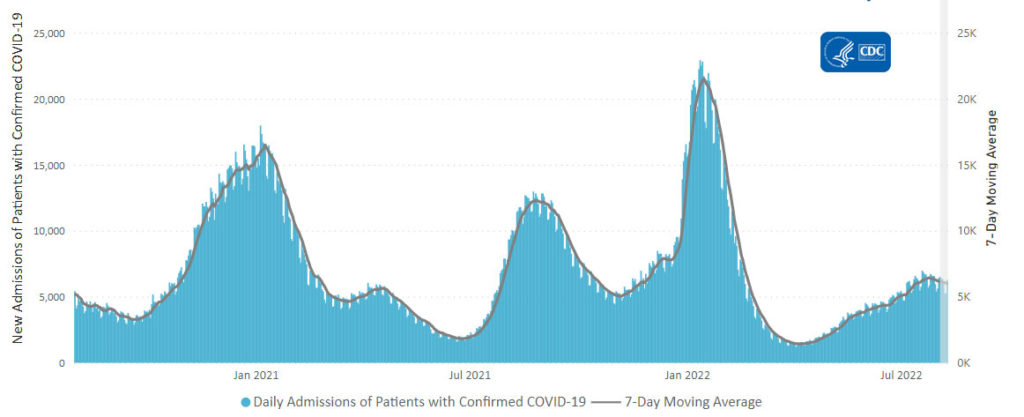
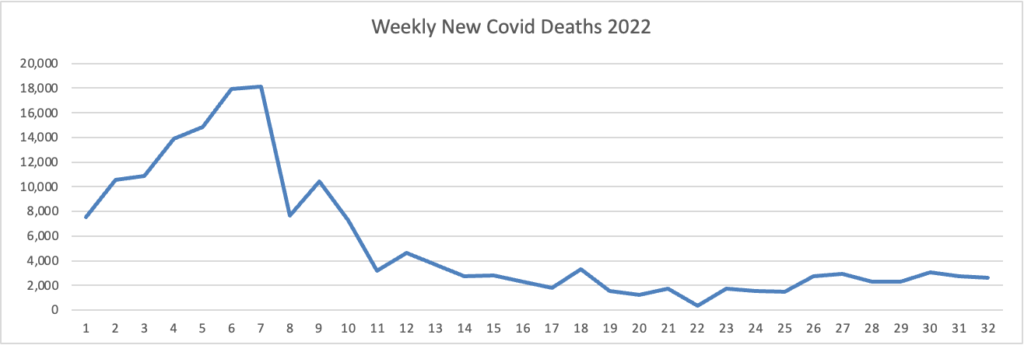
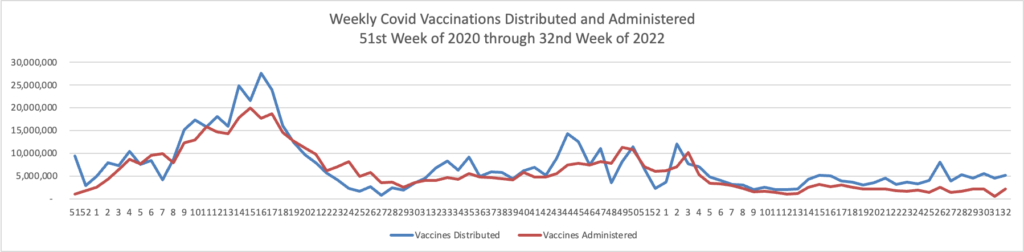
From the Omicron and unusual viruses front —
BioPharma Dive reports
Moderna has joined Pfizer in asking the Food and Drug Administration for emergency authorization of a new COVID-19 booster shot adapted to the virus strains now dominant in the U.S.
The biotech’s reformulated shot targets both the original coronavirus strain and the BA.4/BA.5 omicron subvariants. BA.5 now accounts for almost 90% of cases in the U.S., with versions of BA.4 making up almost all of the rest, according to estimates from the Centers for Disease Control and Prevention.
Moderna and Pfizer have been working closely with the FDA to design boosters that can better fend off COVID-19, particularly as immunity wanes in people who were last vaccinated many months ago. On Monday, Pfizer announced it had finished its application. Moderna followed a day later.
The Wall Street Journal discusses monkeypox’s symptoms, vaccines and how it spreads:
[I]nfectious-disease experts say patterns of transmission in this outbreak have been consistent with the need for close contact, rather than airborne spread. If the virus could easily spread through airborne transmission, they say many more cases outside the LGBT community would be expected. * * *
Most monkeypox cases in the U.S. have been mild, though some patients have experienced moderate to severe disease with symptoms such as extreme pain and high fevers and have had to be hospitalized. * * *
Most monkeypox cases in the U.S. and globally have, to date, been among men who have sex with men. The CDC released data finding the outbreak is concentrated among men who have had sex with several men. Public-health experts say the current risk to the general population is low.
“At this moment in terms of acquiring monkeypox, I wouldn’t change my behavior,” said Dr. Chin-Hong, noting that the calculation would be markedly different for someone in an at-risk group.
Though a handful of children in the U.S. have contracted monkeypox, infectious-disease experts say the risk to most children is very low.
In other public health news
- Becker’s Payer Issues tells us “Cancer has overtaken musculoskeletal conditions as large employers’ biggest driver of healthcare costs, according to Business Group on Health’s ‘2023 Large Employers’ Health Care Strategy and Plan Design Survey.’ Business Group on Health surveyed 135 employers across various sectors that together cover more than 18 million people between May 31 and July 13, according to the August 23 report.”
- Becker’s Hospital Review ranks the states by average life expectancy.
In judicial news —
- The FEHBlog checked the court docket in the Change Healthcare antitrust case today, and he discovered that the defense rested in the bench trial on August 15, and the Court has scheduled closing arguments for September 8. The bench trial, in this case, pending in the U.S. District Court for the District of Columbia, began on August 1.
- The Wall Street Journal reports this evening.
A federal judge blocked Idaho from enforcing its near-total abortion ban in certain emergency situations, an early victory for the Justice Department in a case it filed this month.
U.S. District Judge B. Lynn Winmill on Wednesday issued a preliminary injunction that prevents the state from enforcing its ban in emergency circumstances where doctors and hospitals deem an abortion is necessary to avoid placing the health of a pregnant patient in serious jeopardy. * * *
The ruling was one of two initial tests for Biden administration efforts to require abortion access for emergencies in states that have moved to heavily restrict the procedure. A separate ruling from Texas, issued late Tuesday, went against the administration.
In that case, a federal judge in Lubbock ruled that hospitals and doctors for now aren’t required to abide by the administration’s guidance requiring emergency abortion care.
The next stop is the federal appellate courts in both cases.
In U.S. healthcare business news —
Fierce Healthcare informs us
Three years after it began piloting a primary care service for its employees that blended telehealth and in-person medical services, Amazon plans to cease operations of its Amazon Care service.
Amazon announced Wednesday afternoon that it would end Amazon Care operations after December 31. In an email to Amazon Health Services employees, Neil Lindsay, senior vice president of Amazon Health Services, said Amazon Care wasn’t a sustainable, long-term solution for its enterprise customers.
Amazon provided a copy of the email to Fierce Healthcare.
The decision only impacts Amazon Care and Care Medical teams and not Amazon’s other healthcare services.
CEO Andy Jassy has made health care a priority, naming Amazon Care as an example of “iterative innovation” in his first letter to shareholders earlier this year. In July, the company announced plans to buy concierge primary care provider One Medical in a deal valued at approximately $3.9 billion.
If the One Medical deal goes through, it would significantly expand Amazon’s foothold in the nearly $4 trillion healthcare market, specifically in the competitive primary care market.
One Medical markets itself as a membership-based, tech-integrated, consumer-focused primary care platform. The company operates 188 offices in 29 markets. At the end of March, One Medical had 767,000 members.
The deal also gives Amazon rapid access to the lucrative employer market as One Medical works with 8,000 companies.
This news suggests to the FEHBlog that Amazon is confident that the One Medical acquisition will close.
Kaiser Health News offers an enlightening story on unregulated dietary supplements.
Dietary supplements, which include a broad range of vitamins, herbs, and minerals, are regulated by the FDA. However, they are classified as food and don’t undergo the rigorous scientific and safety testing the government requires of prescription drugs and over-the-counter medicines.
Lawmakers aren’t proposing to put supplements into the same category as pharmaceuticals, but some say they are alarmed that neither the FDA nor the industry knows how many dietary supplements are out there — making it almost impossible for the government to oversee them and punish bad actors.
The FDA estimates 40,000 to 80,000 supplement products are on the market in the U.S., and industry surveys estimate 80% of Americans use them.