Based on the Centers for Disease Control Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog latest weekly chart of new Covid cases:
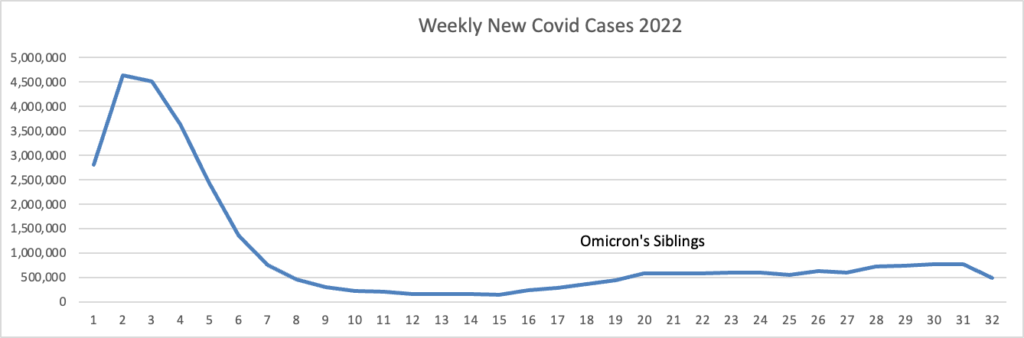
Note: the massive surge on the left is the original Omicron surge, but the label didn’t carry over from the spreadsheet to the FEHBlog.
The CDC’s weekly review of its Covid statistics tells us
As of August 10, 2022, the current 7-day moving average of daily new cases (103,614) decreased 13.8% compared with the previous 7-day moving average (120,151). * * *
CDC Nowcast projections* for the week ending August 13, 2022, estimate that the combined national proportion of lineages designated as Omicron will continue to be 100% with the predominant Omicron lineage being BA.5, projected at 88.8% (95% PI 87.5-90.0%). The national proportion of BA.4 is projected to be 5.3% (95% PI 4.9-5.7%), BA.4.6 is projected to be 5.1% (95% PI 4.1-6.4%), and BA.2.12.1 is projected to be 0.8% (95% PI 0.7-0.9%).
Here’s the CDC’s Daily Trends in Number of New COVID-19 Hospital Admission chart from its weekly review.
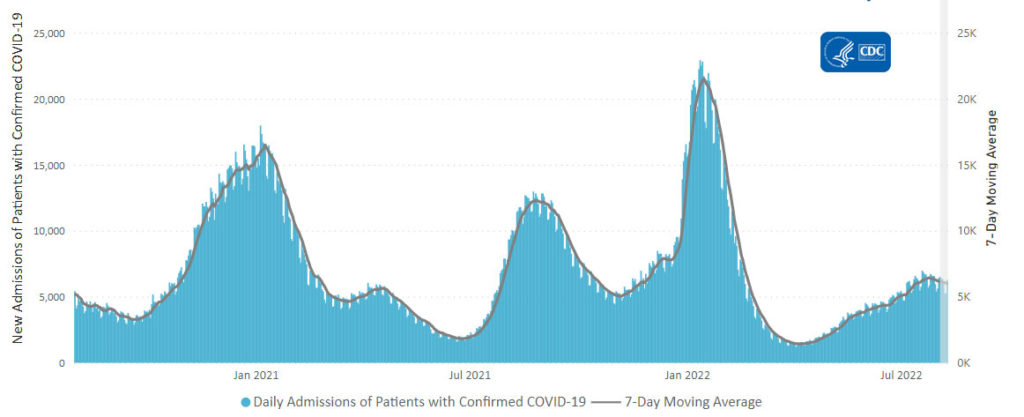
The CDC’s weekly review adds, “The current 7-day daily average for August 3–9, 2022, was 6,003. This is a 2.6% decrease from the prior 7-day average (6,163) from July 27–August 2, 2022.”
Here’s the FEHBlog latest daily chart of new Covid deaths for 2022:
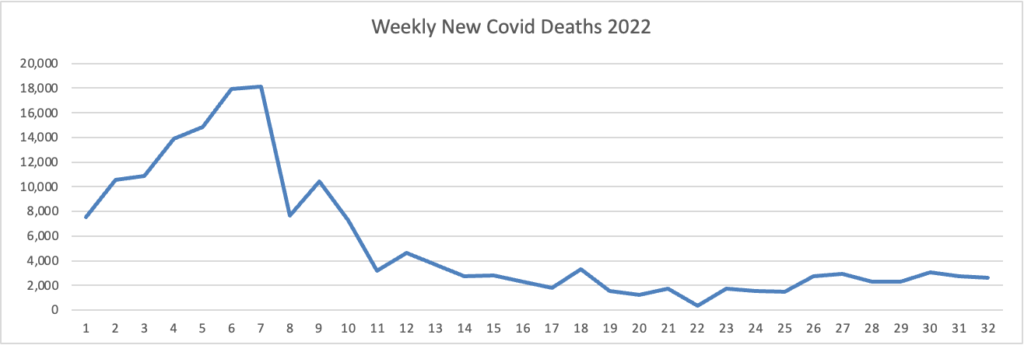
Unfortunately, both labels fell off this chart. The missing label under the horizontal axis 3 -5 reads Omicron, and the missing label over weeks 14 – 17 reads Omicron’s siblings. The CDC’s weekly review adds, “The current 7-day moving average of new deaths (400) has decreased 6.7% compared with the previous 7-day moving average (429).”
New cases, new admissions and new deaths have plateaued and are trending down.
Here’s the FEHBlog’s weekly chart of Covid vaccinations distributed and administered from the first week of the Covid vaccination era in December 2020 to this week.
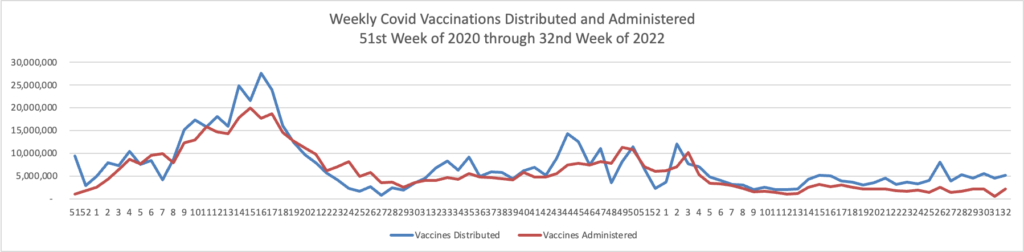
Administered vaccines jumped back up to over 2 million this week. The CDC’s weekly review adds.
Overall, about 262.0 million people, or 78.9% of the total U.S. population, have received at least one dose of vaccine. About 223.5 million people, or 67.3% of the total U.S. population, have been fully vaccinated.* Of those fully vaccinated, about 107.9 million people have received a booster dose,** but 50.0% of the total booster-eligible population has not yet received a booster dose.
MedPage Today informs us
COVID-19 mRNA vaccines have been safe for pregnant women, according to observational data from a large Canadian study.
In fact, the pregnant women reported fewer serious health events than non-pregnant women in the 7 days following vaccination and a similar number of events as a control group of unvaccinated pregnant respondents, as researchers led by Manish Sadarangani, DPhil, of the BC Children’s Hospital Research Institute in Vancouver, reported in The Lancet Infectious Diseases.
The Wall Street Journal reflects on yesterday’s CDC easing Covid restrictions intended to shut down the disease, a state which will not happen anytime soon.
Federal health officials’ move this week to relax pandemic precautions gave business leaders the momentum many have been looking for to return to pre-Covid behaviors.
The new guidelines, issued Thursday by the Centers for Disease Control and Prevention, generally bring the federal guidance in line with policies that had already shifted at companies, schools and public transportation, among other settings. The agency said it no longer recommends that people quarantine after being exposed to the virus, as long as they don’t feel sick, get tested after five days and wear a high-quality mask around others for 10 days.
Many executives and city leaders who had been struggling to break pandemic work-from-home habits see this as a boost to their halting efforts to bring people back into the workplace. They say that previous CDC recommendations made it difficult to enforce their policies, since one exposure could send an entire team home.
It’s worth noting that the CDC issued the revised guidance even though its Spring 2022 innovation based on community levels of the Covid is blinking red.
Overall, 51 out of 52 jurisdictions* had high- or medium-level counties this week. Nevada is the only jurisdiction to have all counties at low Community Levels.
To check your COVID-19 Community Level, visit COVID Data Tracker. To learn which prevention measures are recommended based on your COVID-19 Community Level, visit COVID-19 Community Level and COVID-19 Prevention.
The American Hospital Association calls our attention to another aspect of the Administration’s revised guidance:
The Food and Drug Administration yesterday advised people who get a negative result from an at-home COVID-19 antigen test to test themselves again after 48 hours to reduce the risk of missing an infection and spreading the virus to others.
“Today’s recommendations are based on the latest study results from people with likely omicron infection showing that repeat testing after a negative at-home COVID-19 antigen test result increases the chance of an accurate result,” the agency said
Govexec brings us up to date on the ongoing litigation against the federal government’s Covid vaccination mandate
Remember the vaccine mandate for federal contractors that President Biden issued last September?
Well, several legal challenges are ongoing: there have been six injunctions issued, one of which was national, and the federal government has appealed them all, as outlined in a post from the law firm Bradley Arant Boult Cummings LLP last week.
Ambika Biggs, a partner at the law firm Hirschler who specializes in government contracting, told Government Executive earlier this week the matter is likely to be appealed to the Supreme Court, especially if there is a circuit split on decisions.
These cases really “come down to the interpretation of the Federal Property and Administrative Services Act, which is just called the Procurement Act” and “whether President Biden had authority under that act to issue the executive order that put the federal contractor mandate in place,” said Biggs. The main argument she’s seen in the pleadings is that “there wasn’t a close enough nexus between wanting an economical and efficient system for procurement and the actual vaccine mandate,” and the mandate was more about public health, which is usually under states’ jurisdiction.
The FEHBlog does not expect this issue to get to the Supreme Court.
From the monkeypox front, Beckers Payer Issues recounts seven payer reactions to the announcement of a public health emergency.
From Capitol Hill, the American Hospital Administration reports
The House today voted 220-207 [along party lines] to pass the Senate’s [budget reconciliation bill] (H.R. 5376), sending it to President Biden for his signature. Passed by the Senate Sunday, the roughly $700 billion social spending package would extend for three years the American Rescue Plan Act’s expanded subsidies for coverage purchased through the Health Insurance Marketplaces; allow Medicare to negotiate prices for insulin and certain other drugs; implement tax changes and spending subsidies to address climate change; and reduce the deficit, among other provisions.
The International Foundation of Employee Benefit Plans offers six aspects of the legislation that are relevant to employers and health plan sponsors.
While the prescription drug provisions of the law are tied only to Medicare Parts B and D, employers and plan sponsors that provide retiree health care benefits, especially through employer group waiver plans (EGWPs), will want to be familiar with this legislation and its impact. They may wish to discuss changes with their advisors to see how their plans may be affected.
That’s good advice.
From the U.S. healthcare costs front, the benefits consulting firm Mercer announced
US employers expect health benefit cost per employee to rise 5.6% on average in 2023, according to early results from Mercer’s National Survey of Employer-Sponsored Health Plans 2022, which launched June 22 this year and remains open.
While significantly higher than the increase of 4.4% projected for 2022, the 2023 increase lags overall inflation, which is currently running at about 9% (see Figure 1). According to Sunit Patel, Mercer’s Chief Actuary for Health and Benefits, “Because health plans typically have multi-year contracts with health care providers, we haven’t felt the full effect of price inflation in health plan cost increases yet. Rather it will be phased in over the next few years as contracts come up for renewal and providers negotiate higher reimbursement levels. Employers have a small window to get out in front of sharper increases coming in 2024 from the cumulative effect of current inflationary pressures.”
From the U.S. healthcare business front, Becker’s Payer Issues reports
Humana has completed the sale of a 60 percent stake in its subsidiary Kindred at Home’s hospice and personal care business to the private equity firm Clayton, Dubilier & Rice. The divisions have been restructured into a standalone company, according to an Aug. 11 Humana news release. The $2.8 billion deal was first announced in April. “Humana will continue to support the long-term success of these operations through our minority ownership and ongoing strategic partnership,” CFO Susan Diamond said in the news release.
Health Care Service Corp. has signed an agreement to purchase Trustmark Health Benefits from employee benefits firm Trustmark for an undisclosed amount. HCSC, the parent company of BCBS Illinois, Montana, New Mexico, Oklahoma and Texas, said in an Aug. 11 news release the acquisition would allow the company to serve more members that desire more customizable health benefits. Trustmark Health Benefits provides voluntary benefits, fitness management products and small group plan administration. The Lake Forest, Ill.-based company said no changes to benefits for existing members will occur when the deal is expected to close later this year.
Fierce Healthcare adds
UnitedHealthcare is rebranding one of its fastest-growing health plan designs. Surest plans, first launched in 2016 as Bind, are available nationwide for self-funded employers and in 11 states for fully insured plans. UHC said it plans to make Surest available as an option for fully insured customers in five more states by the end of the year. Surest plans have the fastest growth rate among its employer-sponsored plan designs, the insurance giant said Thursday.
