Happy Pi Day!

From Washington, DC
- The American Hospital Association (AHA) News lets us know,
- “The Senate March 14 approved by a vote of 54-46, a continuing resolution to fund the government through Sept. 30. The House passed, by a vote of 217-213, the bill earlier in the week. President Trump is expected to sign the measure.
- “The bill extends certain key health care provisions that were set to expire at the end of March, including eliminating Medicaid disproportionate share hospital cuts, and extending the enhanced low-volume adjustment and Medicare-dependent hospital programs; key telehealth waivers; the hospital-at-home program; the Work Geographic Index Floor program; and add-on payments for ambulance services.”
- Upshot — No government shutdown.
- Fierce Healthcare adds,
- “A last-ditch effort to pass a bipartisan healthcare package pushed aside at the end of 2024 that includes telehealth extensions and pharmacy benefit manager reform, reverses doc pay cuts and addresses the opioid crisis has failed.
- “That legislation was brought to the floor by Senators Ron Wyden, D-Oregon and Bernie Sanders, I-Vermont, March 14 ahead of a key procedural vote [on the continuing resolution]. The senators’ bill required unanimous consent, or it would not advance. Sen. Rick Scott, R-Florida, quickly rejected the bill.”
- Per a Senate news release,
- “Today, the Senate overwhelmingly voted to pass the Halt All Lethal Trafficking of (HALT) Fentanyl Act. The bipartisan legislation, led by Judiciary Chairman Chuck Grassley (R-Iowa), Health, Education, Labor and Pensions Chairman Bill Cassidy, M.D. (R-La.) and Sen. Martin Heinrich (D-N.M.), would permanently classify fentanyl-related substances before their temporary Schedule I status expires on March 31, 2025.
- “Last month, the HALT Fentanyl Act was passed out of the Senate Judiciary Committee by a bipartisan vote of 16-5. Attorney General Pam Bondi has endorsed the legislation. President Trump’s Office of Management and Budget has confirmed that, if Congress passes the bill in its current form, the president will sign it. The legislation now heads to the House of Representatives.
- “The HALT Fentanyl Act is a critical step towards ending the crisis that’s killing hundreds of thousands of precious American lives. I thank my Senate colleagues for passing this bill with broad, overwhelming support. I urge my House colleagues to swiftly pass the Senate version of this battle-tested, bipartisan bill to save lives, advance research and support our brave men and women in blue,” Grassley said.: * * *
- “Download bill text HERE and a fact sheet HERE.”
- The AHA News tells us,
- “The Senate Finance Committee March 14 held a confirmation hearing on Mehmet Oz’s nomination for administrator of the Centers for Medicare & Medicaid Services. Oz, a doctor and former television show host, indicated that some of his priorities in the position, if confirmed, would be to reduce health care spending by improving poor health, increasing use of technology, incentivizing providers, and stopping wasteful spending, fraud and abuse. The committee will soon schedule a vote on whether to advance his nomination to the full Senate.”
- The Wall Street Journal adds,
- “Mehmet Oz, the Trump administration’s nominee to lead the Medicare agency, said some actions by private insurers in the Medicare Advantage program amounted to fraud and cheating, and he intended to go after them as head of the Centers for Medicare and Medicaid Services.
- “His comments, during his Senate confirmation hearing on Friday, were focused on industry practices that can boost insurers’ Medicare Advantage payments by documenting more diagnoses in their patients. Medicare Advantage insurers get higher payouts when patients have certain conditions.
- “There’s a new sheriff in town,” said Oz, a heart surgeon known for his long-running television show. He criticized “upcoding” in Medicare Advantage and said, “I pledge if confirmed I will go after it.”
- Per Senate news releases,
- “Sen. Chuck Grassley (R-Iowa), a senior member and former chairman of the Senate Finance Committee, laid out his health care priorities during a hearing to consider Dr. Mehmet Oz’s nomination to be Administrator of the Centers for Medicare and Medicaid Services (CMS). Oz committed to supporting Grassley’s efforts to lower prescription drug costs, strengthen rural health care, help kids with exceptional medical needs, preserve transitional health plans and improve the agency’s responsiveness to Congress.”
- and
- “At a U.S. Senate Finance Committee hearing to consider the nomination of Dr. Mehmet Oz to be Centers for Medicare and Medicaid Services (CMS) Administrator, Chairman Mike Crapo (R-Idaho) highlighted Dr. Oz’s wealth of firsthand experience as an accomplished physician and his clear vision for creating a healthier nation. Crapo and Dr. Oz discussed how he would address the nation’s chronic disease epidemic, as well as how he might approach reforming payment programs to improve efficiency.
- “Crapo concluded the hearing with, “There is no doubt you are qualified to serve as the next Administrator of [CMS], and I look forward to voting in favor of your nomination and am urging all of my colleagues to do the same.”
- The AHA News informs us,
- “The Medicare Payment Advisory Commission March 13 released its March report to Congress that includes recommendations for hospital and other Medicare payment systems for fiscal year 2026. Among the recommendations, MedPAC urged Congress to update the base payment rate for hospitals by current law plus 1%.
- “An update above current law is necessary given the combination of providers’ continued financial pressures, and almost two decades of sustained and substantial negative Medicare margins,” AHA wrote in a January letter to MedPAC. “Simply put, even after the recommended payment update, Medicare’s payments to hospitals would remain inadequate.”
- Per a Food and Drug Administration news release,
- “Today, a study co-authored by U.S. Food and Drug Administration scientists was released showing the agency’s youth e-cigarette prevention campaign, “The Real Cost,” successfully reduced e-cigarette use among youth. The campaign, which launched in 2018 under the leadership of President Trump, was found to have prevented an estimated 444,252 American youth (age 11 to 17 at study recruitment) from starting to use e-cigarettes between 2023 and 2024.
- “The new study, published in the peer-reviewed scientific journal American Journal of Preventive Medicine, found evidence that the campaign contributed to the nearly 70% decline in e-cigarette use among American youth that has occurred since 2019. According to the National Youth Tobacco Survey, the number of U.S. middle and high school students who currently use e-cigarettes has declined from 5.38 million in 2019 to 1.63 million in 2024, the lowest level in a decade.”
From the judicial front,
- Politico reports,
- “A federal appeals court has given President Donald Trump’s administration the go-ahead to enforce a pair of controversial executive orders that seek to root out diversity, equity and inclusion efforts in federal agencies and government contractors.
- “The three-member appeals panel — including two judges appointed by Democratic presidents — lifted a lower court’s injunction that had put the policy on hold last month.
- The ruling Friday from the panel of the Richmond, Virginia-based 4th Circuit Court of Appeals is not a final decision on the legality of Trump’s anti-DEI policy. It merely allows the government to administer the policy while litigation continues.
- “In separate opinions explaining their votes, the three judges suggested the Trump administration should be allowed to demonstrate that it will abide by anti-discrimination laws and respect First Amendment rights as it implements the executive orders, which Trump issued on the first two days of his new term.”
From the public health and medical research front,
- The Center for Disease Control and Prevention announced today,
- “Seasonal influenza activity remains elevated nationally but has decreased for four consecutive weeks. COVID-19 activity is declining nationally but elevated in some areas of the country. RSV activity is declining in most areas of the country.
- “COVID-19
- “COVID-19 activity is declining nationally but elevated in some areas of the country. Wastewater levels and emergency department visits are at low levels, and laboratory percent positivity is stable. Emergency department visits and hospitalizations are highest in older adults and emergency department visits are also elevated in young children.
- “There is still time to benefit from getting your recommended immunizations to reduce your risk of illness this season, especially severe illness and hospitalization.
- “CDC expects the 2024-2025 COVID-19 vaccine to work well for currently circulating variants. There are many effective tools to prevent spreading COVID-19 or becoming seriously ill.
- “Influenza
- “Seasonal influenza activity remains elevated nationally but has decreased for four consecutive weeks. Data to date suggest the season has peaked, however, flu-related medical visits, hospitalizations, and deaths remain elevated, and CDC expects several more weeks of flu activity.
- “Additional information about current influenza activity can be found at: Weekly U.S. Influenza Surveillance Report | CDC
- “RSV
- “RSV activity is declining in most areas of the country. Emergency department visits and hospitalizations are highest in children and hospitalizations are elevated among older adults in some areas.
- “Vaccination
- “Vaccination coverage with influenza and COVID-19 vaccines is low among U.S. adults and children. Vaccination coverage with RSV vaccines remains low among U.S. adults. Many children and adults lack protection from respiratory virus infections provided by vaccines.”
- The University of Minnesota’s CIDRAP reports,
- “The measles outbreak in Texas has risen by 36 cases, pushing the US case count for the year past the number for all of 2024.
- “The outbreak of the highly contagious virus, which began in late January and is centered in the western part of the state, now stands at 259 cases, according to the latest update from the Texas Department of State Health Services (DSHS). Of those patients, 257 are either unvaccinated or have unknown vaccination status, and 201 are children ages 17 or younger. Thirty-four patients have been hospitalized, with one death in an unvaccinated child who had no known underlying conditions.
- “Eleven counties to date have reported cases, but two thirds of the cases (174; 67%) are in Gaines County, which has one of the highest rates of school-aged children in Texas who have opted out of at least one vaccine. The county is home to a large Mennonite community with low vaccination rates.
- “DSHS officials said they have determined that three of the case-patients previously listed as vaccinated were not vaccinated. Two had received their measles, mumps, and rubella (MMR) vaccine doses 1 to 2 days before their symptoms started and after they had been exposed to the virus. The third had a vaccine reaction that mimicked a measles infection and has been removed from the case count.”
- FYI, the red rectangle on the map of Texas is Gaines County.
- The University of Minnesota CIDRAP also notes,
- “The US Food and Drug Administration (FDA) yesterday announced the strains it recommends manufacturers include in seasonal flu vaccines for the 2025-26 flu season, and [as usual] they mirror recommendations announced by the World Health Organization (WHO) last month.”
- Gallup reports,
- “Americans’ assessments of their mental and physical health are the least positive they have been in Gallup’s 24-year trend, reflecting a decadelong decline that began around 2013 and accelerated sharply with the onset of the COVID-19 pandemic in 2020.
- “Three in four U.S. adults in Gallup’s latest annual reading rate their mental health (75%) and, separately, their physical health (76%) as either “excellent” or “good.” This contrasts with a record-high 89% rating their mental health positively as recently as 2012, and a high of 82% for physical health in 2003.
- “As fewer Americans have rated their mental and physical health positively, most of the change has been in the percentages rating each aspect “excellent” — shrinking to 31% for mental health and 24% for physical health.”
- The National Science Foundation points out,
- “A team of researchers led by the recipient of a U.S. National Science Foundation Faculty Early Career Development grant has developed a new storage method for protein-based drugs that could potentially eliminate the need for refrigeration of important medicines. Using an oil-based solution and a molecule acting as a coating to enclose the proteins in these drugs, researchers demonstrated a technique to prevent the proteins from degrading rapidly — a protection that traditionally requires refrigeration.
- “The research is led by Scott Medina at Pennsylvania State University and published in Nature Communications. It demonstrates a possible practical application to eliminate the need to refrigerate hundreds of life-saving medicines like insulin, monoclonal antibodies and viral vaccines.
- “The work could eventually reduce the cost of refrigerating such drugs throughout the supply chain and enable greater use of protein-based therapies where constant refrigeration isn’t possible, including military environments.
- “Over 80% of biologic drugs and 90% of vaccines require temperature-controlled conditions. This approach could revolutionize their storage and distribution, making them more accessible and affordable for everyone,” says Medina.”
- Per a National Institutes of Health news release,
- “Researchers at the National Institutes of Health (NIH) have determined that dermatitis resulting from topical steroid withdrawal (TSW) is distinct from eczema and is caused by an excess of an essential chemical compound in the body. Scientists from NIH’s National Institute of Allergy and Infectious Diseases (NIAID) identified treatments that could be studied in clinical trials for the condition based on their potential to lower levels of the chemical compound—called nicotinamide adenine dinucleotide (NAD+), a form of vitamin B3. The findings were published today in the Journal of Investigative Dermatology.
- “Dermatitis is characterized by inflammation, itching, or burning sensations on the skin, and can result from various conditions including TSW and eczema. Eczema, also known as atopic dermatitis, is a common cause of dermatitis and affects 10 to 30% of children and 2 to 10% of adults each year in the United States. Topical steroids—specifically glucocorticoids or topical corticosteroids—have long been used as a first-line treatment for dermatitis caused by eczema because the drugs are safe, effective, easy to apply, and considered well-tolerated.” * * *
- “The scientists provisionally established criteria that can be used by health care providers to identify TSW in people. People who have stopped topical steroid treatment and meet the criteria may be diagnosed by practitioners as having TSW. The researchers suggest that patients identified as having TSW could be treated using the proposed mitochondrial complex I-blocking drugs.
- “The results of this study may help practitioners identify TSW in patients and work towards developing safe and effective treatments. According to the researchers, more research is needed to determine whether all patients with TSW have an excess of NAD+, or if there are other features that define TSW. Additionally, the diagnostic criteria will help health care providers and researchers to better understand the prevalence of TSW and evaluate the effects of using topical steroids.”
From the U.S. healthcare business front,
- The New York Times reports,
- “It’s easy to make a medical case for blockbuster weight loss drugs like Wegovy and Zepbound, which have been shown to prevent heart attacks and strokes and save lives.
- “But for the employers and government programs being asked to pay for the medications, the financial case for them is less clear. Are the drugs’ benefits worth their enormous cost?
- “The answer right now is no, according to a new study published on Friday in the journal JAMA Health Forum, by researchers at the University of Chicago.
- “To be considered cost effective by a common measure used by health economists, the price of Novo Nordisk’s Wegovy would need to be cut by over 80 percent, to $127 per month, the researchers concluded. And Eli Lilly’s Zepbound would be cost effective only if its price fell by nearly a third, to $361 per month. (Zepbound warranted a higher price, the researchers said, because it produced greater benefits in clinical trials.)”
- Per STAT News,
- “Biotech company Altimmune announced in an investor call yesterday that it will study its obesity drug, which targets receptors of the GLP-1 and glucagon hormones, in alcohol use disorder, as mounting evidence supports the potential of GLP-1 drugs to help with addiction.
- “Altimmune executives think that their molecule, called pemvidutide, could be particularly helpful for liver conditions, since there are glucagon receptors located in the liver. The company has already been studying the drug in weight loss and in the liver disease MASH, and are now expanding into alcohol-related conditions.
- “The company plans to start a study in alcohol use disorder in the second quarter of this year, and another study in alcohol-related liver disease in the third quarter.
- “Eli Lilly has also started studying mazdutide, a GLP-1/glucagon drug it’s developing with Chinese biotech Innovent, in alcohol use disorder. Lilly CEO Dave Ricks has said the company also plans to test obesity drugs in other areas of addiction, like nicotine and drug use disorders.
- “Novo Nordisk is running a study of its obesity drugs in alcohol-related liver disease but has not yet started any trials in addiction.”
- Per Kaufmann Hall
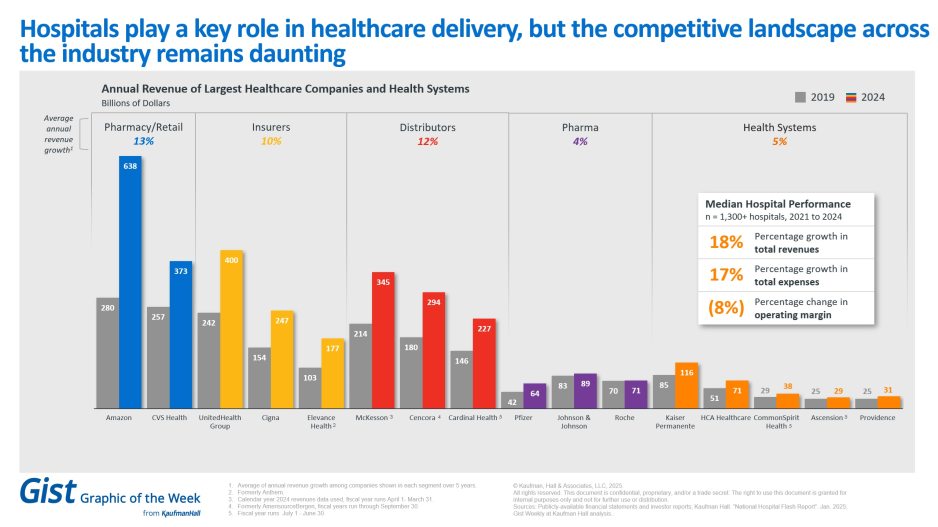
- Per Beckers Hospital CFO Report,
- As healthcare organizations continue to navigate shifting financial and operational landscapes, new data from Kaufman Hall and Strata highlights significant trends in physician productivity, compensation, and costs.
- In the fourth quarter, median work relative value units per full-time physician varied across specialties, with medical specialties leading at 7,139 wRVUs. Physician compensation followed a similar pattern, with surgical specialists earning the highest median paid compensation at $491,000 per FTE.
- Despite an encouraging increase in net revenue per physician, rising expenses remain a challenge. These figures underscore the mounting financial pressures and workforce challenges facing healthcare organizations. For executives, balancing rising costs, evolving productivity demands, and compensation structures will be critical in sustaining financial stability and operational efficiency.
- Beckers Hospital Review points out ten drugs poised to be top sellers next year.








