Tuesday’s Tidbits

From Capitol Hill, Roll Call reports that the House Speaker Nancy Pelosi negotiated a Democrat legislative victory when the House narrowly approved a rule adopting the $3.5 trillion budget blueprint and agreeing to hold a floor vote on the Senate’s bipartisan, $1 trillion infrastructure bill no later than September 27, 2021.
Leadership is hoping to have the reconciliation package, which committees have a Sept. 15 deadline to assemble, ready for floor action around the same time as the infrastructure bill with the goal of passing both by the end of September, House Majority Leader Steny H. Hoyer told reporters on a press call Tuesday afternoon.
How soon the reconciliation package is ready “will dictate to some degree the flow of legislation,” the Maryland Democrat said. “We have not got a hard view on sequencing.”
Federal News Network informs us about three items for federal employees to watch in the bipartisan infrastructure bill.
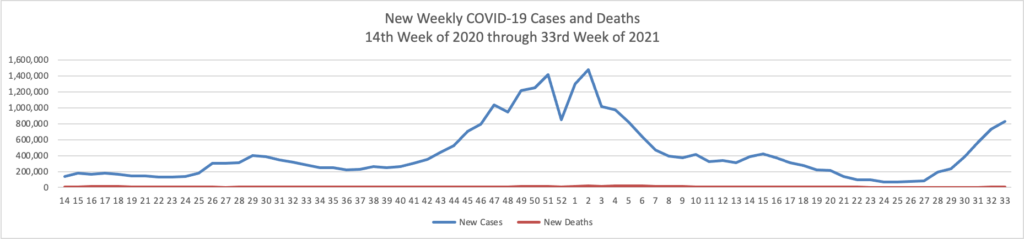
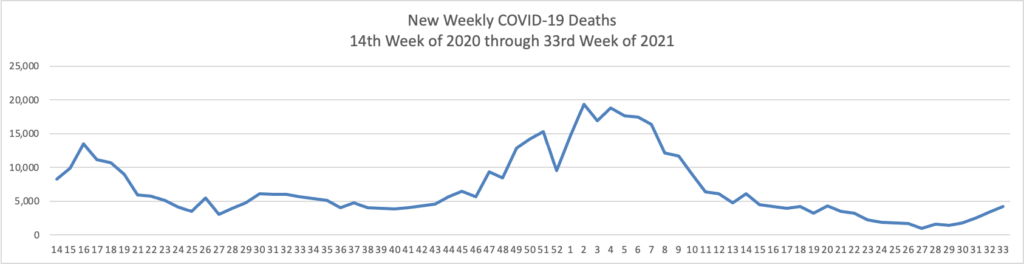
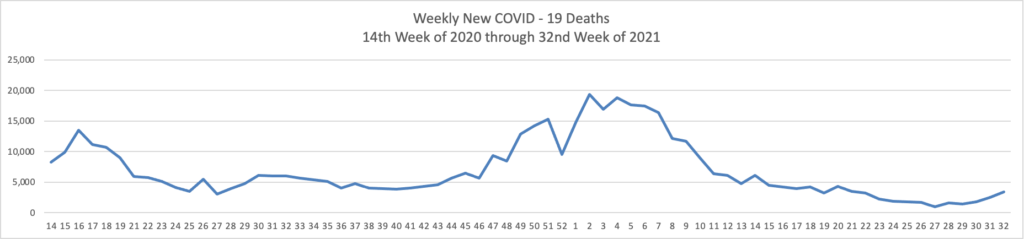
From the Delta variant front, the American Hospital Association tells us
The Centers for Disease Control and Prevention today released research highlighting two important trends emerging from the COVID-19 pandemic regarding vaccines’ current effectiveness. The first, which looked at breakthrough infections among fully vaccinated front-line health care workers, found that, following the emergence of the SARS-CoV-2 delta variant, there has been a moderate reduction in the effectiveness of COVID-19 vaccines in preventing infection in this group. However, the authors note that “this trend should be interpreted with caution because [vaccine efficacy] might also be declining as time since vaccination increases and because of poor precision in estimates due to limited number of weeks of observation and few infections among participants.” Additionally, the CDC notes that even a sustained two-thirds reduction in infection risk underscores COVID-19 vaccinations’ continued importance and benefits in preventing deaths, hospitalizations and the virus’ spread.
The second study reviewed SARS-CoV-2 infections in Los Angeles County over the course of nearly three months, starting in May 2021. Researchers found that approximately three out of four cases were among unvaccinated individuals. Additionally, infection and hospitalization rates among unvaccinated persons were 4.9 and 29.2 times more likely, respectively, than those in fully vaccinated persons, with little change when the delta variant took root as the nation’s dominant virus strain.
The Society for Human Resource Management discusses what the FDA’s full approval for the Pfizer vaccine means for employer COVID-19 policies.
The Wall Street Journal informs us that
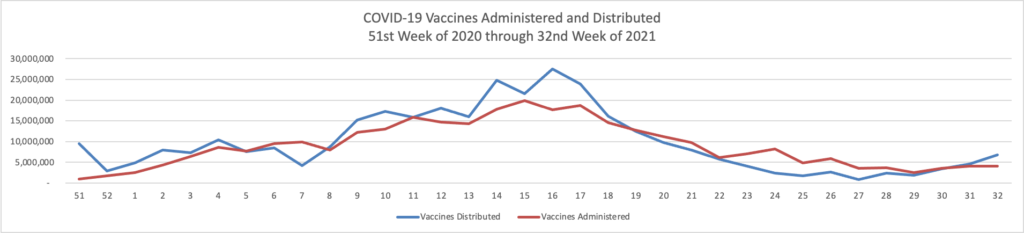
Johnson & Johnson said [over Tuesday night] that a second dose of its [one dose] Covid-19 vaccine was found in a study to generate a strong immune response, justifying a booster shot after eight months. * * * J&J said researchers found antibody levels increased ninefold among people who received a second dose of its vaccine, compared with one month after they received a first dose. * * *
“We look forward to discussing with public health officials a potential strategy for our Johnson & Johnson Covid-19 vaccine, boosting eight months or longer after the primary single-dose vaccination,” [said] Dr. Mathai Mammen, global head of R&D at Janssen Pharmaceutical Cos. of Johnson & Johnson.
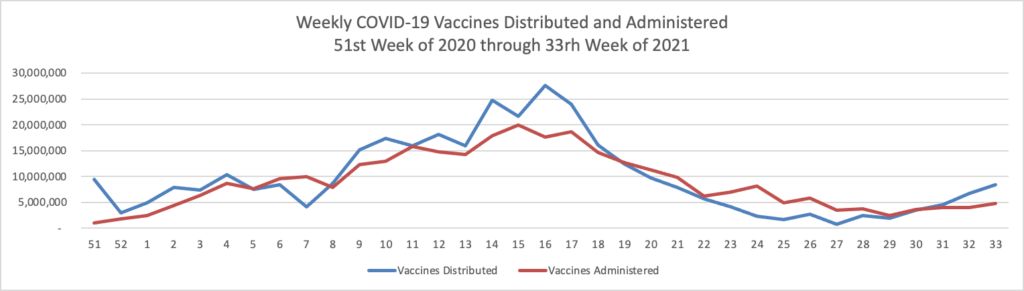
The Biden administration said last week that people ages 18 and older who got the Covid-19 vaccines from Pfizer Inc. or Moderna Inc. should get an extra dose eight months later, reflecting heightened concern over the highly contagious Delta variant and data showing initial immunity to Covid-19 diminishes over time. J&J’s suggested timeline would be in sync with that broader strategy.
Currently boosters are only authorized for immunocompromised people who received two-dose messenger-RNA vaccines. The Food and Drug Administration must authorize extra doses before they can be offered more broadly as recommended by the Biden administration to those 18 and older who received two-dose messenger-RNA vaccines, and it is expected to do so before Sept. 20, when health authorities said messenger-RNA boosters would become available
The CDC’s expert advisory panel on vaccines will meet next week to discuss the Biden administration’s plans for booster shots.
According to the CDC’s website, this virtual ACIP meeting will be held on August 30 and 31.
Finally STAT News reports that
The age at which adults who are overweight or obese should be screened for type 2 diabetes is going down while the prevalence of both forms of the disease is going up among children and adolescents — two developments reported Tuesday that signal a growing burden of these chronic health conditions among Americans.
The U.S. Preventive Services Task Force lowered its recommended age to 35 — down from 40 in its 2015 guidance — to test people with above-normal BMIs for elevated glucose levels that could mean prediabetes or diabetes itself. The new evidence review and recommendations, published in the Journal of the American Medical Association, would make more than 40% of adults eligible for screening, and an estimated one-third will likely meet USPSTF criteria to undertake preventive steps.