Tuesday’s Tidbits

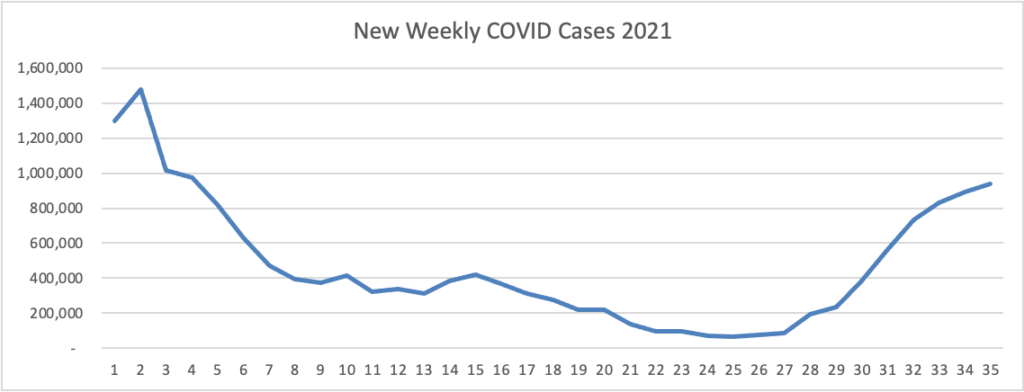
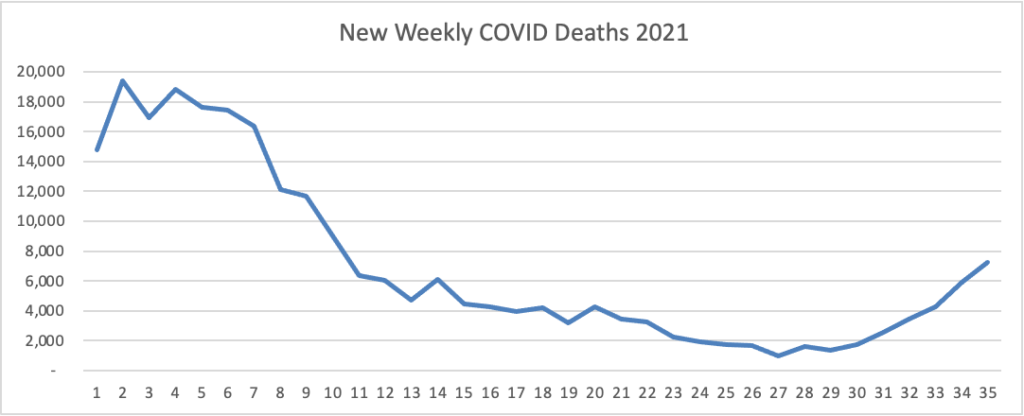
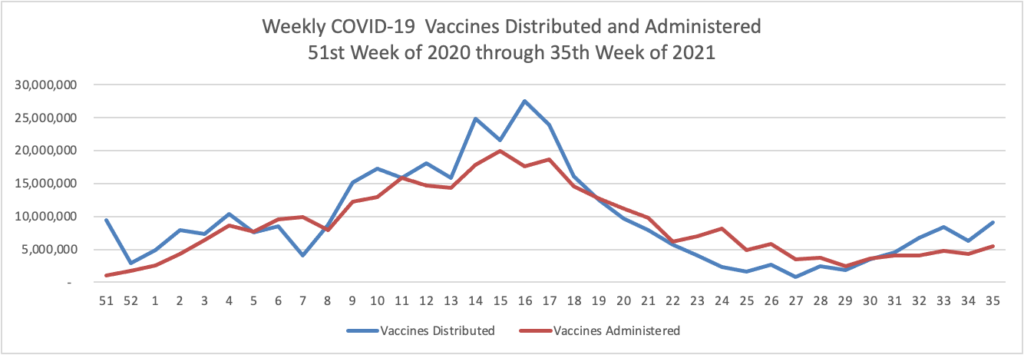
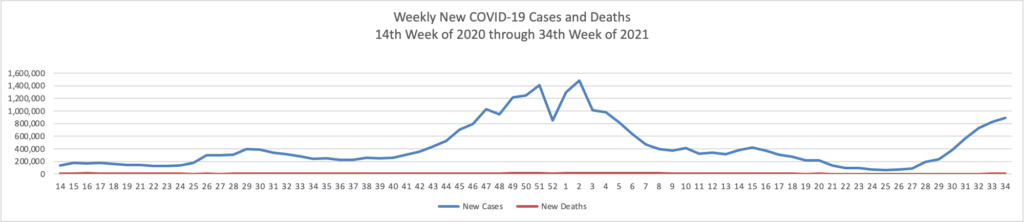
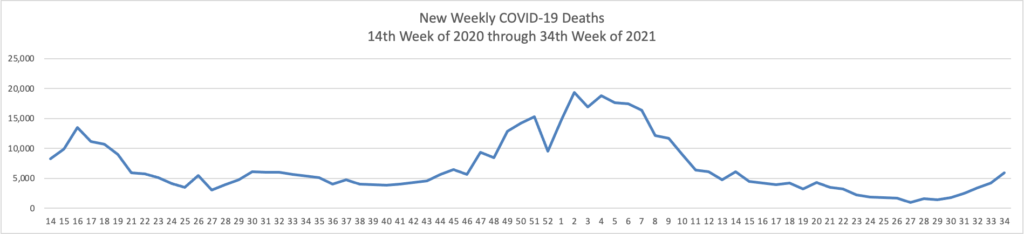
On the Delta variant front and acccording to the CDC’s COVID Data Tracker, the United States reached two data points today — the number of COVID cases now exceeds 40 million and the percentage of Americans aged 18 and over who have received at least one dose of a COVID-19 vaccine reached 75%.
At the end of 2020, the number of cases according to the CDC stood at 20 million. In his blog, National Institutes of Health Director Francis Collins discusses a recent Nature study estimating that “the true number of [COVID] infections by the end of 2020 at more than 100 million [1]. That’s equal to just under a third of the U.S. population of 328 million. This revised number shows just how rapidly this novel coronavirus spread through the country last year. It also brings home just how timely the vaccines have been—and continue to be in 2021—to protect our nation’s health in this time of pandemic.” It also suggests to the FEHBlog that we may to closer to effective herd immunity in some areas of the U.S. than generally thought.
Also David Leonhardt in today’s New York Times tells us about another way to look at the situation.
The C.D.C. reported a terrifying fact in July: Vaccinated people with the Delta variant of the Covid virus carried roughly the same viral load in their noses and throats as unvaccinated people.
The news seemed to suggest that even the vaccinated were highly vulnerable to getting infected and passing the virus to others. Sure enough, stories about vaccinated people getting Covid — so-called breakthrough infections — were all around this summer: at a party in Provincetown, Mass.; among the Chicago Cubs; on Capitol Hill. Delta seemed as if it might be changing everything.
In recent weeks, however, more data has become available, and it suggests that the true picture is less alarming. Yes, Delta has increased the chances of getting Covid for almost everyone. But if you’re vaccinated, a Covid infection is still uncommon, and those high viral loads are not as worrisome as they initially sounded.
How small are the chances of the average vaccinated American contracting Covid? Probably about one in 5,000 per day, and even lower for people who take precautions or live in a highly vaccinated community. * * *
I will confess to one bit of hesitation about walking you through the data on breakthrough infections: It’s not clear how much we should be worrying about them. For the vaccinated, Covid resembles the flu and usually a mild one. Society does not grind to a halt over the flu.
In Britain, many people have become comfortable with the current Covid risks. The vaccines make serious illness rare in adults, and the risks to young children are so low that Britain may never recommend that most receive the vaccine. Letting the virus continue to dominate life, on the other hand, has large costs.
“There’s a feeling that finally we can breathe; we can start trying to get back what we’ve lost,” Devi Sridhar, the head of the global public health program at the University of Edinburgh, told The Times.
Well put, Mr. Leonhardt, as usual.
From the federal employee benefits front, OPM posted on the Federal Register website today a notice of changes to Federal Group Life Insurance premium rates for “Employee Basic Insurance, Option A (most age bands), Option B (most age bands), Option C (most age bands), and Post-Retirement Basic Insurance. These rates will be effective the first pay period beginning on or after October 1, 2021.”
From the tidbits department
- Federal News Network reports that “The White House is proposing billions of dollars in supplemental funding for disaster relief and other programs, which it’s asking Congress to attach to a short-term continuing resolution that will be critical toward avoiding a government shutdown at the end of the month. ‘With the end of the fiscal year rapidly approaching, it’s clear that Congress will need to pass a short term continuing resolution to provide more time for the fiscal 2022 process to unfold,’ Shalanda Young, the Office of Management and Budget’s acting director, said Tuesday in a blog post.”
- Fierce Healthcare informs us that “The American Medical Association [“AMA”] released updates to its medical codes for 2022 with many tied to new technology services and the administration of COVID-19 vaccines. The AMA made 405 changes in the 2022 Current Procedural Terminology code set, including 249 new codes, 63 deletions and 93 revisions. The changes will take effect Jan. 1. ” The CPT is recognized as a HIPAA electronic transaction code set.
- AP News reports that “Four companies in the drug industry said Saturday that enough states had agreed to a settlement of lawsuits over the opioid crisis for them to move ahead with the $26 billion deal. An announcement from the three largest U.S. drug distribution companies and a confirmation from drugmaker Johnson & Johnson, which had previously announced that it would move ahead, came Saturday. That was the deadline for the companies to decide whether there was enough buy-in to continue the settlement plan. * * * Together, the settlements are likely to represent the biggest piece of a string of settlements between companies in the drug industry and state and local governments over the addiction and overdose epidemic in the U.S.”
- Healthcare Dive tells us that “The use of telehealth for patient visits seems to have leveled off at 20% or fewer of all appointments, more than a year and a half after COVID-19 first spurred an unprecedented jump in utilization, according to a new survey from KLAS Research and the Center for Connected Medicine.”
- The AMA discusses “what doctors wish patients knew about a prediabetes diagnosis,” which of course is a fairly common diagnosis in our country.
- The Wall Street Journal continues its series on the Future of Everything in healthcare with an article about sensor studded smart clothes. “From a prescription bra that signals cardiac arrest to a mosquito-proof textile, startups and scientists are developing garments for healthier living.”