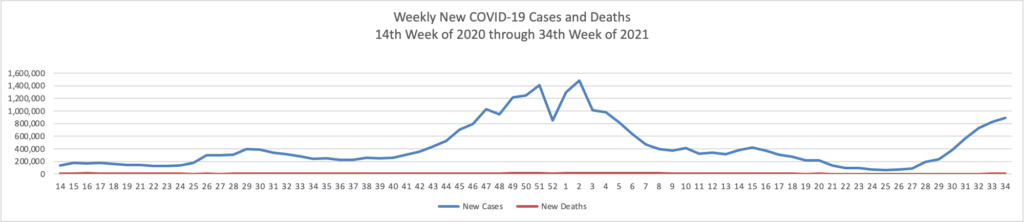
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 34th week of this year (beginning April 2, 2020, and ending August 25, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases significantly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through August 25, 2021):
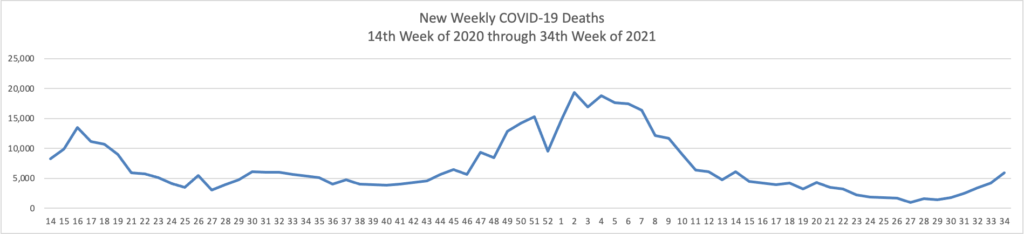
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through August 25, 2021, which also uses Thursday as the first day of the week:

The cases, hospitalizations, and death charts move continue to move in the wrong direction. New vaccinations remain steady as people recognize that the Delta variant is more aggressive than the 2020 wave. If there is any bright side it is that the elderly (age 65 and older) who are at the greatest risk of death from COVID-19 are the most vaccinated group in the U.S. with 81.5% fully vaccinated and another 10% at the first dose stage.
For more stats, here’s a link to the CDC’s weekly interpretative review. “As of August 26, 2021, 203 million people in the United States have received at least one dose of a COVID-19 vaccine. 172 million people are fully vaccinated. That’s 60.8% of the eligible population (12 years and older). * * * COVID-19 vaccines remain the most powerful tool we have against COVID-19, making it critical that all people get vaccinated as soon as they are eligible. To find a vaccine provider near you, visit vaccines.gov or your state or local public health department.”
The CDC also issued its 2021-22 flu season vaccination recommendations today.
Routine annual influenza vaccination is recommended for all persons aged ≥6 months who do not have contraindications. * * * Balancing considerations regarding the unpredictability of timing of onset of the influenza season and concerns that vaccine-induced immunity might wane over the course of a season, particularly for older adults, vaccination is recommended to be offered by the end of October. * * * Children aged 6 months through 8 years who require 2 doses (i.e., children in this age group who have never received influenza vaccine or who have not previously received a lifetime total of ≥2 doses; see Children Aged 6 Months Through 8 Years) should receive their first dose as soon as possible after the vaccine becomes available to allow the second dose (which must be administered ≥4 weeks later) to be received, ideally, by the end of October. Children of any age who require only 1 dose for the season should also ideally be vaccinated by the end of October; vaccination of these children may occur as soon as vaccine is available because there is less evidence to suggest that early vaccination is associated with waning immunity among children compared with adults.
Also from the Delta variant front
- The Numbers columnist in the Wall Street Journal reports that “Medical studies often use thousands of volunteers. But sometimes good things come in small packages—like a handful of people willing to contract a deadly virus. Researchers in the U.K. have deliberately infected 30 volunteers with the virus that causes Covid-19, in the first human challenge study of the disease. Infecting the volunteers—who are healthy, unvaccinated and range in age from 18 to 30—will allow the scientists to observe in real time how the virus attacks the body and, from the moment of exposure, how the immune system responds. * * * [In contrast to a large clinical study involving the use of placebo] a human challenge study of a relatively small number of participants offers precise answers to specific questions, often related to immune response. Today, human challenges conducted under the supervision of institutional review boards are routinely used to research diseases such as influenza, malaria, cholera, salmonella, shigellosis and norovirus.”
- The Journal also reports that British scientists are making progress in carefully growing the Delta variant for use in these human challenges. The U.S. is not conducting human challenges involving COVID-19 at this time.
- The Journal also informs us that “U.S. intelligence agencies are unable to determine conclusively how the Covid-19 pandemic emerged, a summary of a classified report released Friday said.” The article concludes “In a July 27 letter to Mr. Biden, the Democratic and Republican leaders of the Senator Foreign Relations and Intelligence committees urged the president to carry on with the investigation until the intelligence community had reached conclusions on the origin of the pandemic with a high degree of confidence. The letter urged that the inquiry examine what U.S. government funding was provided to the Wuhan Institute of Virology for advanced virus research.”
In other news —
- The Federal Times reports that “Federal employees will get a total 2.7 percent pay raise in 2022, as President Joe Biden informed Congress Aug. 27 that he intends to exercise his authority to determine federal pay rates during a state of emergency.” The increase will breaks down into a 2.2% general increase and a 0.5% locality pay increase.
- Fierce Healthcare tells us that “OptumRx has released its quarterly look at the drug pipeline, and two of the therapies highlighted in the report target fairly common conditions. Finerenone, or the brand name Kerendia, was approved by the Food and Drug Administration on July 9. The drug treats chronic kidney disease and type 2 diabetes. Some 26.8 million Americans have been diagnosed with diabetes, and one in three eventually develop some kind of kidney disease. Bill Dreitlein, senior director of pipeline and drug surveillance at OptumRx, told Fierce Healthcare that the drug will be entering a market where many patients are already treated by low-cost therapies. “With the entrance of this drug, some patients are going to shift from a low-cost treatment to a higher-cost treatment,” he said. The other drugs highlighted in the report are: (1) Atogepant, which is pending a brand name, a drug that treats episodic and chronic migraines; (2) Odevixibat, or the brand name Bylvay, which treats progressive familial intrahepatic cholestasis, a liver disease, and (3) Maralixibat, which also has yet to set a brand name, treats Alagille Syndrome, a rare genetic disease of the liver.
- Healthcare Dive informs us that “COVID-19 hospitalizations continue to rise as coronavirus cases surge across the U.S. This once again puts pressure on hospital operations and will likely put downward pressure on nonprofit hospital margins, according to a new report from Fitch Ratings. In some areas, hospitalizations are higher than they were during previous surges.”
