Weekend update

The House of Representatives and the Senate will be in session this week for limited Committee business and floor voting.
The Wall Street Journal explains
Lawmakers will return to the Capitol this week with a singular focus of passing a sweeping bipartisan spending bill to avert a shutdown and fund the government through September, despite opposition from many House Republicans.
The massive bill is expected to total around $1.7 trillion and could be released as early as Monday. It would fund government agencies and programs and allow those agencies to distribute grants and contracts to the private sector.
Because it is the last piece of legislation that Congress will pass in this session, lawmakers have spent weeks lobbying to attach other bills, including funding for Ukraine, changes to tax policy and a measure to update how Congress deals with disputes over certifying presidential-election results. * * *
The Senate is expected to vote on the bill first. Senate Minority Leader Mitch McConnell (R., Ky.) has been part of the negotiations and set a deadline of Thursday to reach a deal—a day before the money runs out—but he said his patience was limited and that he wouldn’t allow talks to stretch past Christmas.
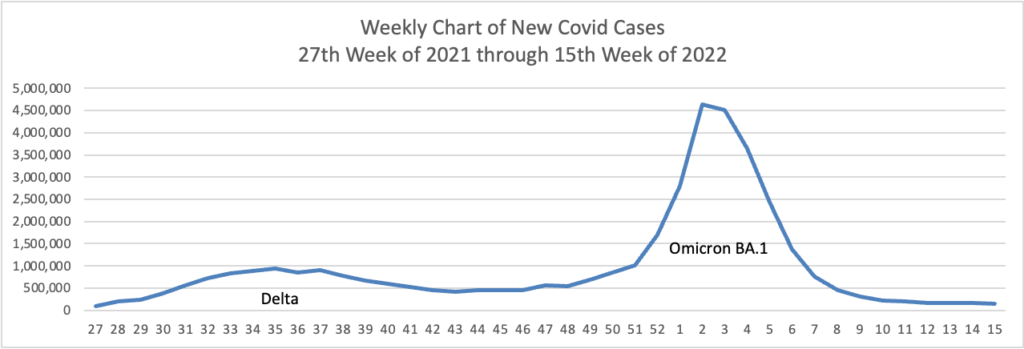
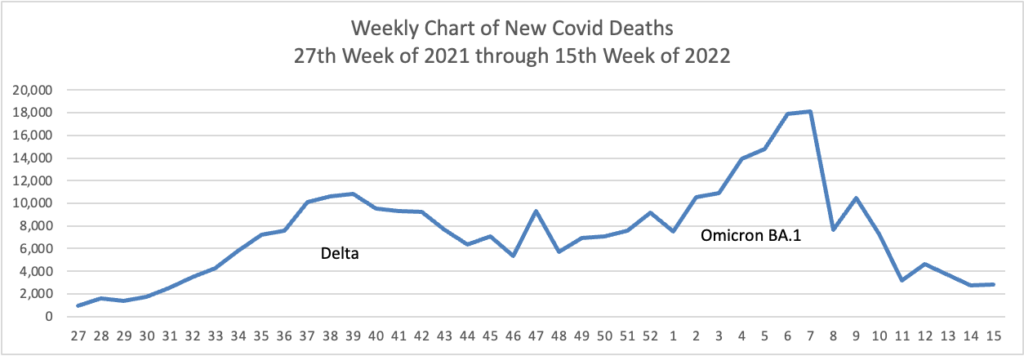
From the Omicron and siblings front, NPR Shots provides insights on Paxlovid.
The Centers for Disease Control and Prevention recommends treatment for patients at risk of severe disease, hospitalization and death, which includes anyone who’s 50 and older (risk increases with age), people who are unvaccinated and people with certain medical conditions, such as obesity, chronic lung disease, heart disease or a weakened immune system.
But exceptions can be made. A colleague who is under 50 told her doctor she was feeling worse each day after her positive COVID test and had a history of pneumonia. The doctor wrote a Paxlovid prescription. * * *
[In addition to you doctor or pharmacist, t]he federal government has a “Test to Treat” locator to see where you can be tested for free and, if you test positive and are eligible, leave with the drug. Spots include community health centers and some pharmacies.
Pharmacies may also send the pills to your home for prescriptions the doctor calls in. Walgreens just announced free Paxlovid delivery via Door Dash and UberEATS; CVS will send it the same day for a fee. * * *
Since Paxlovid has to be taken within five days of symptoms starting to work, you might contact your doctor’s office to find out what steps to take if you test positive and think you need the drug. * * *
Paxlovid is the best option for reducing the risk of severe disease. The last monoclonal antibody treatment for COVID-19 lost its FDA authorization last month because it is ineffective against currently circulating variants. That leaves Paxlovid; remdesivir, which requires an outpatient infusion over three days at a hospital or treatment center; and molnupiravir [the other pill], which studies put at only 30% effective in treating the virus. In addition, some doctors are treating immunocompromised patients with convalescent plasma.
Bloomberg Prognosis discusses expiration dates on at-home Covid tests.
The Food and Drug Administration has extended the shelf-lives of 14 brands of tests. Consumers can look up their specific brand and even the lot number to see the correct expiration dates. Brands including iHealth, from a subsidiary of Andon Health in China, Abbott Laboratories’ BinaxNow and ACON Labs Inc.’s Flowfex now last up to 12 months, 15 months and 21 months, respectively. The FDA advises against using at-home Covid tests past their expiration date.
Health plans and Medicare continue to provide at-home Covid tests at no cost, and the federal government resumes mailing out free at-home Covid tests tomorrow.
From the telehealth front —
mHealth Intelligence tells us
Implementing a telehealth navigator program helped improve video visit attendance, providing clinics with a positive financial return, according to a new study published in JAMA Network Open.
The COVID-19 pandemic dramatically drove up the use of telehealth. Like many other healthcare provider organizations, Boston-based Beth Israel Deaconess Medical Center implemented and scaled telehealth visits. But they found that technical issues could hamper video visits, prompting some video visits to be converted into audio-only visits via the telephone, according to the study authors.
The medical center implemented a patient navigator pilot program to reduce barriers to video visit attendance. Through the program, a patient navigator contacted patients one day before their video visit appointment to provide technical support. The navigator went through the steps required for the patient to connect to their visit and addressed frequently asked questions.
The Wall Street Journal reports
Remote treatment of mental-health problems surged in the pandemic, as in-person treatment became difficult while pandemic-driven isolation increased anxiety and depression.
Digital mental-health companies plunged in, promising to provide millions with access to high-quality care by video, phone, and messaging.
Many of the businesses, however, put a premium on growth. Investor-backed, they deployed classic Silicon Valley tactics such as spending heavily on advertising and expansion while often using contractors instead of employees to control costs. A strategy designed for mundane businesses such as food delivery, the formula can be badly suited to the sensitive activity of treating mental-health problems.
No bueno. The article is focused on stand-alone telemental health services.
In the spirit of the Season, Bloomberg Prognosis tackles the question of “Eggnog Made With Raw Eggs Safe.”
“Eggnog may be safely made at home by using egg substitutes, whole, liquid or pasteurized eggs,” Darin Detwiler, a food-safety expert at Northeastern University, says. “These products need no further cooking to kill harmful bacteria.”
Pasteurized eggs are gently heated in their shells to a high-enough temperature to kill any bacteria without cooking the egg. They are pretty widely available, though the texture isn’t always exactly the same as an unpasteurized egg.
If you are making eggnog the old-fashioned way, Detwiler has some advice for that, too.
“Cook the egg mixture to 160℉ and refrigerate it quickly in several small containers,” he says. “Then it will cool quickly.”
Jingle bells, all.