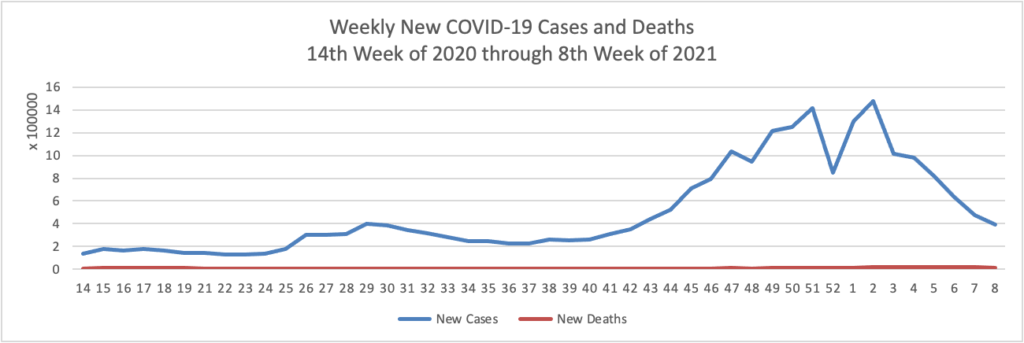
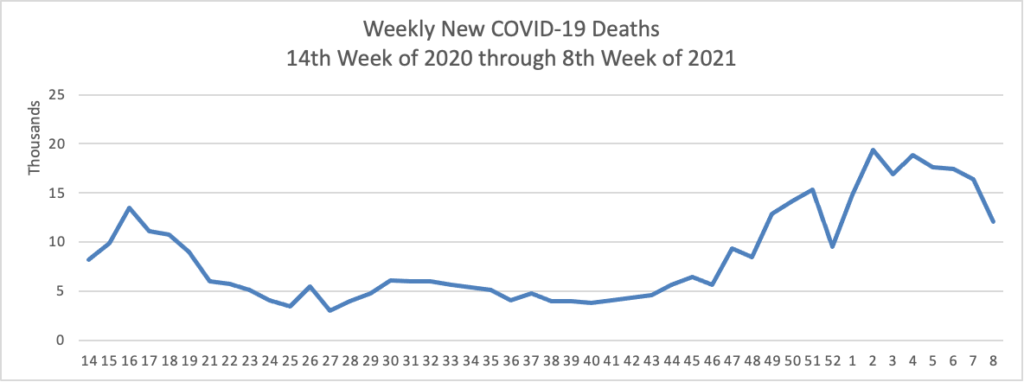
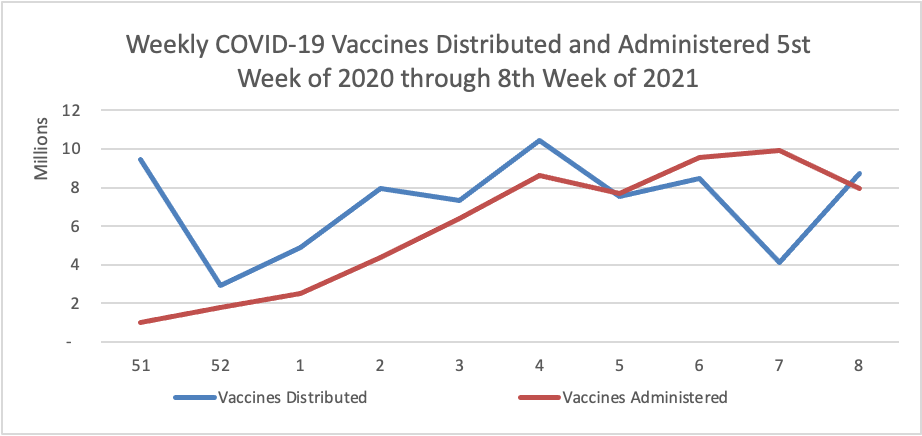
Midweek update

From Capitol Hill, STAT News reports that Senate leaders have reached an agreement to extend a Medicare pay bump for health care providers through 2021, a major lobbying win for hospitals.”
The Wall Street Journal reports two Senate confirmations in healthcare positions:
The Senate [today] confirmed Dr. Rachel Levine as assistant health secretary, making her the first openly transgender federal official approved by the Senate.
The vote was 52 to 48, largely along party lines, with GOP Sens. Lisa Murkowski of Alaska and Susan Collins of Maine joining all 50 Democrats.
The pediatrician and former Pennsylvania secretary of health helped steer the state’s response to Covid-19. She has also worked to increase awareness of equity issues that the LGBT community faces and is a professor of pediatrics and psychiatry at Penn State College of Medicine.
and
Yesterday, the Senate approved Vivek Murthy, President Biden’s pick for surgeon general, by a 57-to-43 vote, marking his second stint in the post, which he held from 2014 through 2017. Dr. Murthy, who was co-chairman of Mr. Biden’s Covid-19 advisory board, has said he would use his position to provide science-based guidelines for ending the coronavirus pandemic.
From the COVID vaccine front, Medscape informs us that
White House officials said at a briefing Wednesday they are still anticipating updated vaccine data from AstraZeneca, after federal officials called Tuesday’s release of interim phase 3 data from the company “outdated information.”
“Right now, AstraZeneca is getting back with the Data and Safety Monitoring Board and will likely come out with an updated statement,” said Anthony Fauci, MD, a top COVID-19 official and chief of the National Institute of Allergy and Infectious Diseases, the agency that complained to the pharmaceutical company that their current information was “incomplete.”
Andy Slavitt, senior White House adviser for COVID-19 response, added: “Our takeaway is the importance of transparency and trust…. I would urge us not to focus on the process of the last couple days, but instead to focus on what really matters, which is what happens when these applications for these candidates are submitted to the FDA.”
FLASH — The Washington Post reported at 10:30 pm Wednesday night that
An updated company analysis of the coronavirus vaccine developed by AstraZeneca and the University of Oxford showed that the two-shot regimen was robustly effective — 76 percent at preventing symptomatic illness — according to a news release from the drugmaker late Wednesday.
The finding, only slightly lower than results announced days earlier, underscores that the vaccine being widely used by many countries appears to be a powerful tool to help end the pandemic. No severe cases of illness were reported in study volunteers who received the vaccine. Among people 65 and older, the vaccine was 85 percent effective, the company reported.
Yesterday, the FEHBlog watched a Wall Street Journal interview with Mr. Slavitt as part of the WSJ’s Health Forum. The FEHBlog really enjoyed this WSJ video featuring reporter Joanna Stern with a COVID vaccine hunter from New Jersey. It’s certainly worth five and half minutes of your day.
HR Dive reports that
Employers should offer paid sick leave to employees with “signs and symptoms” following COVID-19 vaccination, according to guidance updated March 16 by the Centers for Disease Control and Prevention.
Employers should consider on-site vaccination programs if they have a large workforce with predictable schedules and enough space to run a clinic that meets social distancing requirements, CDC said. Employers that choose to offer vaccinations should record each offer and employees’ decisions. Employers should consider off-site vaccination if they are a small- or medium-sized organization lacking the resources to host a vaccination clinic, it said.
The agency also said that whether an employer may require COVID-19 vaccinations is a matter of state or other applicable law but noted that exemptions may apply: Medical exemptions for people who are at risk for an adverse reaction because of an allergy to one of the components used in the vaccine or a medical condition; and religious exemptions for people who reject being vaccinated because of their religious beliefs.
In healthcare business news, Fierce Healthcare lets us know that
Uber is ramping up its prescription delivery business by teaming up with software company ScriptDrop. The ride-share giant will be the default delivery service for ScriptDrop pharmacies in 37 states and will eventually expand to others.
ScriptDrop works with some of the top grocery chains, pharmacy chains and health systems in the U.S., including Albertsons, Jewel-Osco, Safeway and Vons. Through the tie-up with Uber, those pharmacies will be able to leverage the company’s technology to deliver more prescriptions to more customers.
From the report front, the FEHBlog noticed
- CVS Health has made public its Health Trends Report 2021.
- The Foley & Lardner law firm has released its 50-State Survey of Telehealth Commercial Insurance Laws.
Finally March 22 to 28, 2021, is National Drug and Alcohol Facts Week. “Held since 2010, NDAFW brings teens and scientific experts together to discuss the scientific facts about drugs, as well as their potential health effects on teen bodies and brains. ”