Midweek Update

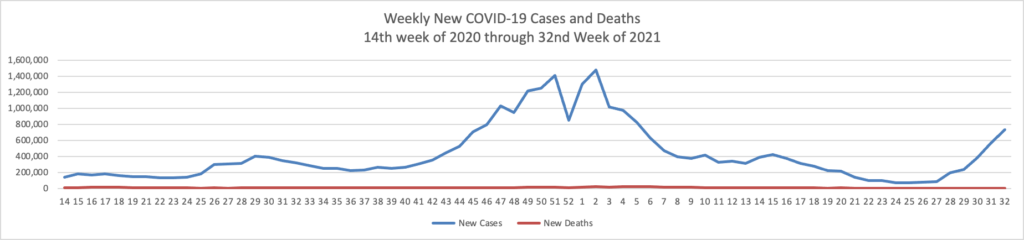
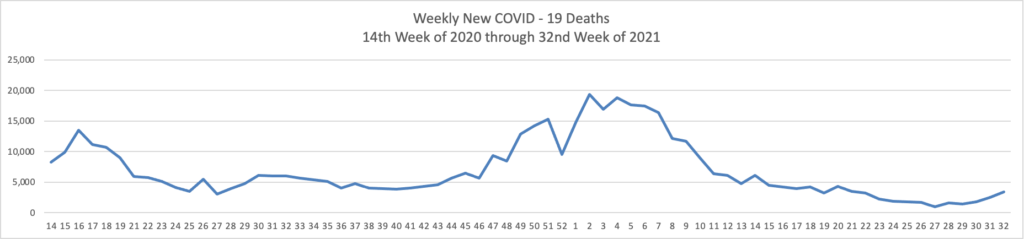
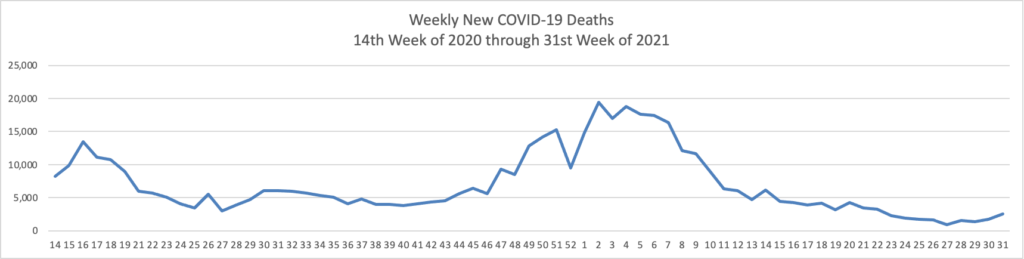
From the Delta variant front –
- In a joint statement, a group of high ranking HHS public health experts explained today that
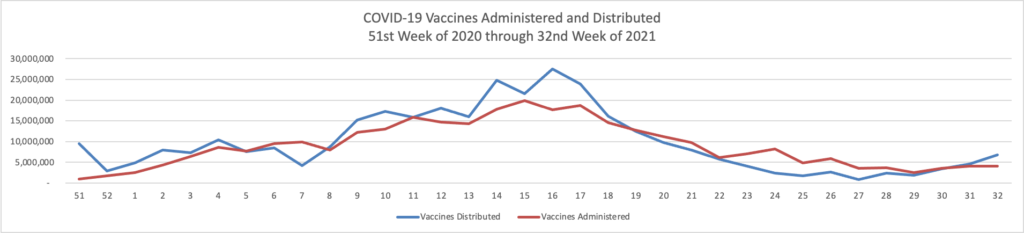
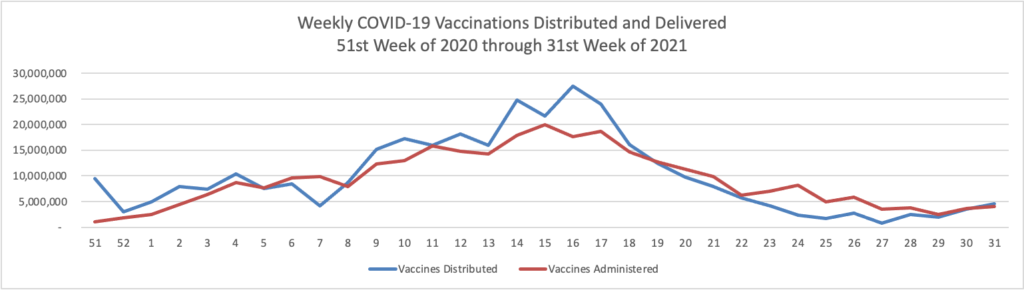
“We have developed a plan to begin offering [COVID-19 vaccination] booster shots this fall subject to FDA conducting an independent evaluation and determination of the safety and effectiveness of a third dose of the Pfizer and Moderna mRNA vaccines and CDC’s Advisory Committee on Immunization Practices (ACIP) issuing booster dose recommendations based on a thorough review of the evidence. We are prepared to offer booster shots for all Americans beginning the week of September 20 and starting 8 months after an individual’s second dose. At that time, the individuals who were fully vaccinated earliest in the vaccination rollout, including many health care providers, nursing home residents, and other seniors, will likely be eligible for a booster. We would also begin efforts to deliver booster shots directly to residents of long-term care facilities at that time, given the distribution of vaccines to this population early in the vaccine rollout and the continued increased risk that COVID-19 poses to them.
“We also anticipate booster shots will likely be needed for people who received the Johnson & Johnson (J&J) vaccine. Administration of the J&J vaccine did not begin in the U.S. until March 2021, and we expect more data on J&J in the next few weeks. With those data in hand, we will keep the public informed with a timely plan for J&J booster shots as well.”
The FEHBlog will be in line for his third dose of the Pfizer vaccine when the time comes.
- The Wall Street Journal reports that “Early data from Israel suggests a booster shot of Pfizer Inc.’s Covid-19 vaccine can significantly improve immunity in those aged 60 and above, as the U.S. and other countries plan additional doses to increase protection against the highly infectious Delta variant.”
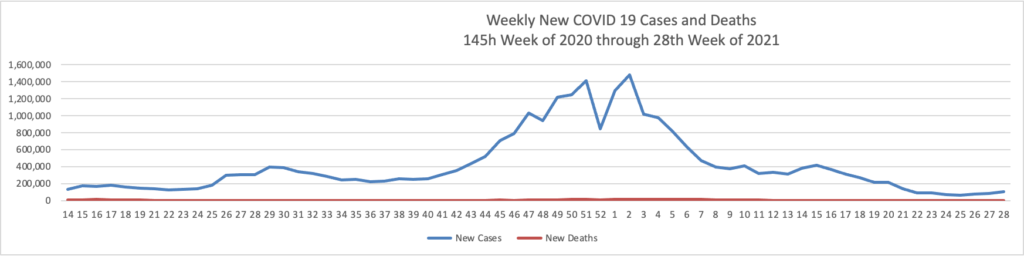
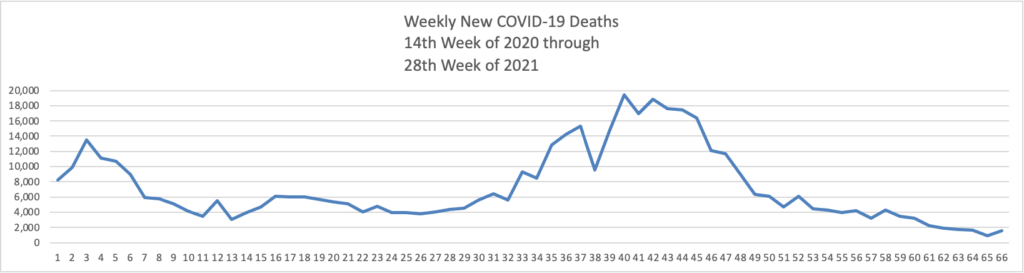
- Health Affairs reports on a study suggest”[ing] that the early COVID-19 vaccination campaign was associated with reductions in COVID-19 deaths. As of May 9, 2021, reductions in COVID-19 deaths associated with vaccines had translated to value of statistical life benefit ranging between $625 billion and $1.4 trillion.” The smartest move that the government made was to prioritize the elderly who suffered the most deaths during the pre-vaccination era of COVID-19.
From the federal employee vaccination screening program front, the Safer Federal Workforce task force issued a set of FAQs on COVID-19 testing employees, contractors and visitors who cannot attest to receiving a COVID-19 vaccination. The FEHBlog was pleased to read that the FAQs impose the testing cost on the agencies, not on the FEHB Program, which is the proper legal outcome under the federal CARES Act (unnumbered FAQ 3). Federal News Network makes its own observations on the Testing FAQs here.
In healthcare utilization news, Healthcare Dive reports that
- More than one in 10 adults ages 16 to 64 said they delayed or went without needed healthcare services due to virus fears in the past 30 days, an April survey from the Urban Institute funded by the Robert Wood Johnson Foundation found.
- One in 10 parents delayed seeking care for their children for that reason, according to the report published Wednesday.
- Hispanic and Black adults, along with adults with lower incomes, reported delaying care at higher rates than other groups. Adults with chronic health problems were also more likely than those without such conditions to say they went without needed care.
It’s worth noting that this survey was conducted during the month that vaccinations became widely available and before the Delta variant broke out.
In other healthcare news
- Govexec reports that “Officials at the Centers for Disease Control and Prevention announced on Wednesday that the agency is launching a new organization to focus on disease forecasting. The Center for Forecasting and Outbreak Analytics will be a hub for research and innovation aimed at mitigating the effects of future disease threats. Its launch comes as the federal government continues to fight the coronavirus pandemic and now the rapidly spreading Delta variant. It will build on current modeling efforts at the agency. * * * The center’s initial funding will come from the $1.9 trillion American Rescue Plan enacted in March for coronavirus relief.”
- The NCQA Blog discusses the hospital at home movement in the U.S. “Humana Home Solutions Vice President Dr. Amal Agarwal estimated that up to 35% of Medicare Advantage spending might be addressable at home. As Mayo Clinic Platform President John Halamka explained, hospital at home also “brings the family back into wellness.” This matters because family involvement affects patient satisfaction.” Interestingly the experts explained that hospital at home care is best suited for mid-level acuity patients, not folks who need the ICU or folks who don’t require hospitalization.
Dr. Halamka used an accessible and memorable analogy to outline the long-term possibilities for hospital at home.
He explained that the tractor manufacturing company John Deere transformed itself into a data company by covering its tractors with sensors. The sensors report back information about the weight and volume of crops that customers harvest—soybeans, for example. The predictive value of the information reported to John Deere is so high that the data are now used to forecast soybean prices.
Likewise, Americans are filling their homes and strapping to their bodies millions of behavioral and biometric sensors.
“We are instrumenting homes with sensors to gather patient data that we can use to understand not only that patient’s progression, but aggregating and analyzing that data [to] understand the progression of similar patients,” said Halamka.
Well put, Doctor.
- In support of extending initiatives like this to rural areas of the country, the Department of Health and Human Services announced today “key investments that will strengthen telehealth services in rural and underserved communities and expand telehealth innovation and quality nationwide. These investments—totaling over $19 million—are being distributed to 36 award recipients,” such as “Telehealth Centers of Excellence (COE) program: $6.5 million is being awarded to 2 organizations to assess telehealth strategies and services to improve health care in rural medically underserved areas that have high chronic disease prevalence and high poverty rates. The Telehealth COEs will be located in academic medical centers and will serve as telehealth incubators to pilot new telehealth services, track outcomes, and publish telehealth research. The COEs will establish an evidence-base for telehealth programs and a framework for future telehealth programs.’