Midweek Update

From the COVID-19 front
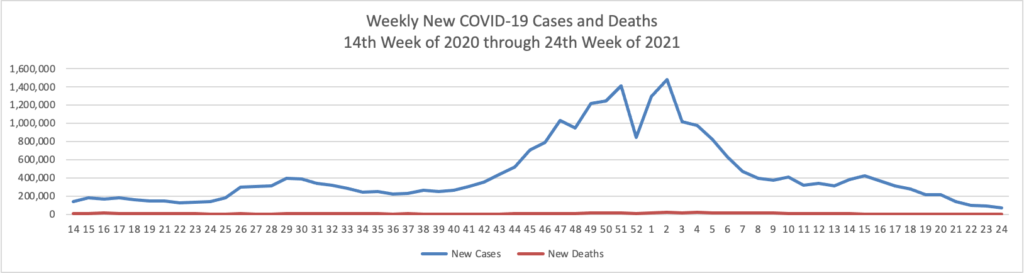
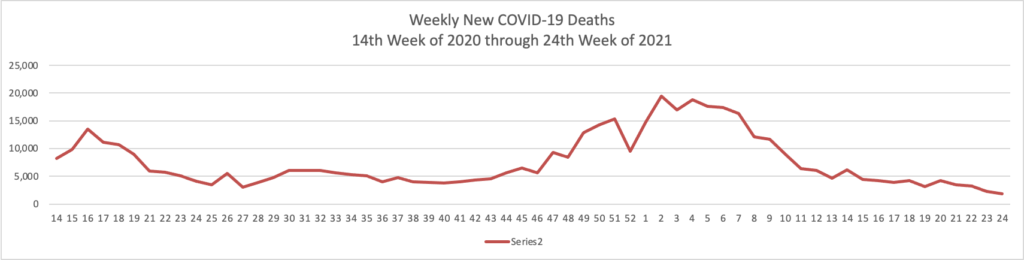
- The Wall Street Journal reports that “The highly transmissible Delta variant has become the dominant strain of the Covid-19 virus circulating in the U.S., according to federal data. It is spreading rapidly as communities loosen pandemic restrictions and officials struggle to reach unvaccinated people. The Delta variant, also known as B.1.617.2, made up 51.7% of Covid-19 infections in the two weeks ended July 3, according to genetic sequences from positive Covid-19 tests submitted to the U.S. Centers for Disease Control and Prevention.”
- The Journal adds that “Covid-19 vaccines available in the U.S. protect against the Delta variant, but the virus is of great risk to people who aren’t vaccinated, according to public-health and infectious-disease experts. * * * Warm weather is helping to keep numbers of new cases down, and infections are likely to rise again in the fall, said Dr. Paul Sax, clinical director of the Division of Infectious Diseases at Brigham and Women’s Hospital in Boston. ‘Last summer we got a little bit overconfident,’ he said. ‘I don’t want us to make the same mistake again this time. We need to push as hard as we can to get as many people vaccinated as possible.’”
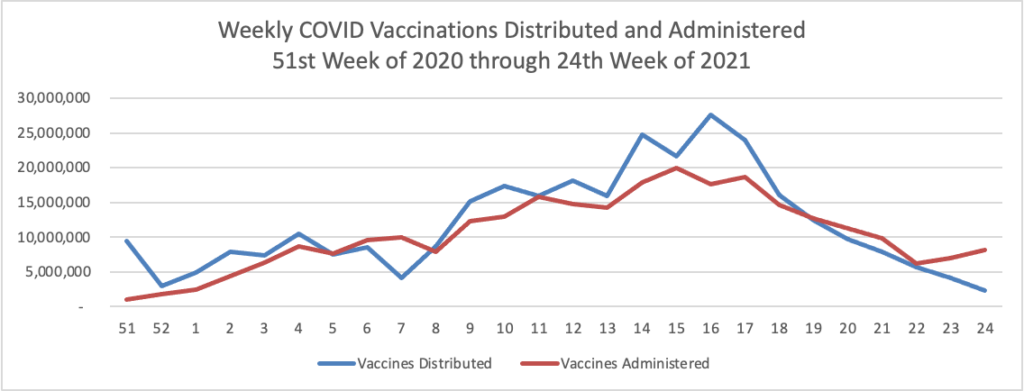
- AHIP updates us on the progress made by its Vaccination Community Connectors program. “The COVID-19 pandemic has been a fundamental part of Americans’ lives for 15 months and counting. America cannot afford to lose any more time in achieving community immunity. Public-private partnerships have helped to support our communities through the crisis this far. By extending those partnerships through secure sharing of data about who has been immunized, we can better target every outreach and connection to put an end to the pandemic and get back to the moments we all miss. For more information, read our white paper with Blue Cross Blue Shield Association (BCBSA) and the Association for Community Affiliated Plans (ACAP).”
- The FEHBlog noticed today that the OPM Inspector General has posted his semi-annual report to Congress for the period ended April 30, 2021, and OPM’s management response thereto. The Inspector General’s report includes an update of his earlier assessment of the pandemic’s impact on the FEHB Program. With all due respect, the FEHBlog finds the OIG’s assessment unnecessarily pessimistic but it’s for the readers to form their own opinions.
From the innovation front
- Fierce Pharma reports that “Many pursuits have been put on hold during the coronavirus pandemic. But biopharmaceutical innovation isn’t one of them. In 2020, the FDA approved 53 new drugs, the second-most in a single year, after 2018’s bounty of 59. And the momentum has continued through the first half of 2021. With the FDA endorsing its 29th novel drug on June 30, the industry was slightly ahead of last year’s pace. * * * n terms of treatment areas, it is of little surprise that oncology accounts for 12 of this year’s approvals. That figure represents 44% of all new drug approvals this year, an even higher rate than in 2020 when 20 of 53 new drugs were in the oncology class. * * * The FDA’s roundups of 2021’s novel drug approvals can be found here and here.”
- Employee Benefit News informs us that “Employers have a new tool in their arsenal to help employees reach a healthier weight and reduce their healthcare costs. DayTwo, a precision medicine company, has released new outcomes from its employer and health plan nutrition programs, to tackle high-risk and high-cost metabolic conditions, like obesity, Type 2 diabetes and pre-diabetes. The program provides users with a microbiome screening, which measures how the body digests food, in order to offer AI-powered nutritional plans tailored to their needs. After one year, employees who used the DayTwo program lost an average of 19 pounds and reduced their body mass index by 3.3 points, according to a release. The program is meant to reduce the reliance on medication and help employees with obesity and Type 2 diabetes lose weight naturally.”
- mHealthIntelligence tells us that “While many healthcare providers are just now getting into the remote patient monitoring arena, Ochsner Health has scaled its platform to a national level, and is now monitoring more than 20,000 people in health plans across the country. And still, says Julie Henry chief operating officer for the New Orleans-based health system’s digital medicine department, ‘we’re learning lessons each and every day.’ That’s one of the guiding principles behind a connected health service that is seeing immense growth in the wake of the coronavirus pandemic, which has pushed many health systems to shift healthcare services from the hospital to the home. It’s a work in progress for everyone, from those deploying the technology to those paying for it. And there isn’t a hospital, clinic or practice out there that isn’t learning something new.”
In other healthcare news
- The Federal Register announced today the last Thursday’s No Surprises Act interim final rule will be published in the July 13 issue. Publication triggers the sixty day public comment period which should end on Monday September 13 (as the 60 day period ends on Saturday September 11.)
- Beckers Hospital Review reports that “Amazon Care, the e-commerce giant’s new healthcare venture, has approached several big health insurers in an effort to expand coverage of its services, Insider reported July 7. The healthcare venture reportedly talked to Aetna, Premera Blue Cross and Blue Cross Blue Shield of Massachusetts, according to people familiar with the discussions.”