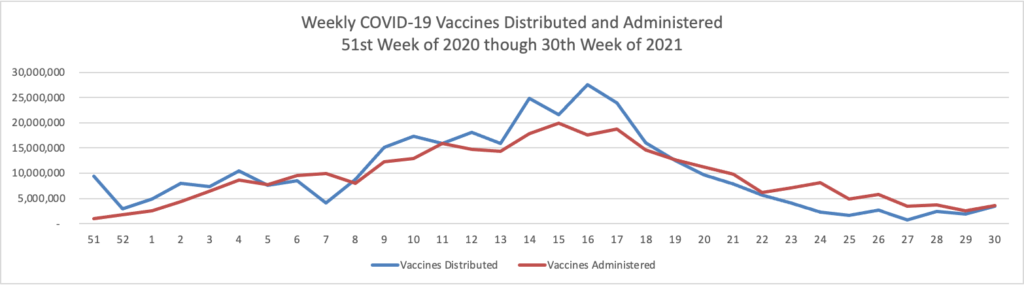
Here comes Open Season

Today OPM issued its first notice about the Federal Benefits Open Season which will run this year from Monday November 8 through Monday December 13.
[Benefits Administration Letter] BAL 21-401 provides guidance on the upcoming Federal Benefits Open Season for the Federal Flexible Spending Account Program (FSAFEDS), Federal Employees Dental and Vision Insurance Program (FEDVIP) and the Federal Employees Health Benefits (FEHB) Program. Attached to this BAL is a sample email and “Circle Round Your Benefits” flyer. This BAL and the attachments will be posted on our website at www.opm.gov/retirement-services/publications-forms/benefits-administration-letters/.
The BAL makes a couple of points worth noting and includes a timeline which also is partially excerpted below:
Employees find Open Season fairs a valuable resource for getting Open Season information. Due to COVID-19, we strongly encourage you to assess how in-person benefit fairs will be impacted. Consider other ways to provide information to employees such as virtual events, webcasts, or webinars. Many health plans host virtual events to provide information about Open Season to their enrollees and others. You may contact health plans for ideas and suggestions on providing information.
| 2022 rates announced and posted on OPM website | Late September |
| BAL 21-403 Significant Plan Changes | Anticipated Issue Date: Early-to Mid-October |
| Open Season information posted on OPM website | Early November |
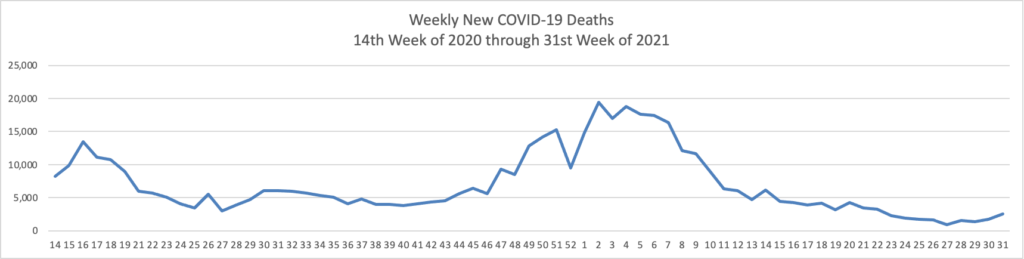
From the Delta variant front
The Washington Post informs us that
The Food and Drug Administration has scheduled a key meeting on coronavirus boosters with its outside advisers for Sept. 17 — just a few days before the Biden administration’s planned starting date for an extra-shot campaign.
The session, which will be public, could add much-needed clarity and transparency to a decision-making process that some people have criticized as confusing. But it also could fuel more controversy over an administration position some experts regard as premature.
Of course, the Biden administration could reduce the time pressure by postponing its “plan starting date.”
On a related note, The Wall Street Journal reports that
The Food and Drug Administration is considering whether to authorize a lower dose of Moderna Inc.’s Covid-19 vaccine for boosters than the dose given in the first two shots, people familiar with the deliberations said.
Moderna said Wednesday it is asking the FDA to authorize a 50 microgram dose, half the dosage of the first two shots. Some in the government are leaning toward authorizing the 100 microgram dose, the people said, because of concerns a lower-dose booster might not offer a durable enough boost to counter fast-changing variants of Covid-19.
No final decision has been made, the people said, as the FDA is still reviewing data from studies that tested boosters using the different doses. People who have seen the data said both doses produce a strong immune response.
Presumably a smaller dose would reduce side effects.
CNBC brings us up to date on the other COVID-19 variants of concerns besides Delta.
The CDC is monitoring four variants “of concern,” including delta, which was first detected in India and is the most prevalent variant currently circulating in the U.S.; alpha, first detected in the U.K.; beta, first detected in South Africa, and gamma, first detected in Brazil. A variant of concern is generally defined as a mutated strain that’s either more contagious, more deadly or more resistant to current vaccines and treatments.
It’s also keeping a close watch on four other variants of interest — including lambda, first identified in Peru [and presumably mu, first identified in Columbia] — that have caused outbreaks in multiple countries and have genetic changes that could make them more dangerous than other strains.
Also from the FEHB front —
Fedweek offers short but accurate guidance about OPM’s FEHB disputed claims review process.
Health Payer Intelligence informs us that “The Alliance of Community Health Plans (ACHP) is urging the federal government to take action and lower prescription drug prices with a set of recommended actions.” In addition to recommendations for Medicare Part D and the biosimilar market, ACHP makes broader recommendations such as
Targeting drug companies’ unjustifiable raising of drug prices. At the beginning of 2021, 735 drugs prices increased up to 10 percent without reason. Prescription drug prices often increase faster than the inflation rate, therefore ACHP recommended that drug manufacturers should have to provide rebates for drug price increase above the inflation rate. Drug companies should also have to follow a price transparency rule that would require manufacturers to report and justify price increases, ACHP stated.
The federal government [should] encourage the use of transparent fee-based pharmacy benefit managers (PBMs). Traditional PBMs are typically not transparent about rebates, which can encourage high-cost drug use, whereas transparent fee-based PBMs pass rebates and discounts onto payers and earn revenue through a clear administrative fee.
ACHP may be interested to know that OPM imposed a strict regime of transparent pricing on experience rated FEHB plans ten years ago. Transparent pricing, which OPM continues to fine tune, facilitates OPM audits of PBMs but does not generate substantial new savings for carriers. Instead, transparent pricing shifts PBM fees from a share of rebates and such to “a clear administrative fee” at levels which simply were not charged before 2011. It is important to add that in 2010 OPM also mandated that experience rated carriers rebid their PBM contracts triennially and those market competitions have generated substantial new savings for carriers. Also as the FEHBlog has mentioned, if OPM were to allow FEHB carriers to offer Medicare Part D EGWPs, as Congress authorized in 2003, the resulting savings, in the FEHBlog’s estimation, would generate blockbuster new savings that would actually lower FEHB premiums.
From the miscellany department —
- MedPage Today tells us that “The FDA approved the injectable, long-acting atypical antipsychotic paliperidone palmitate (Invega Hafyera), a twice-yearly treatment for schizophrenia in adults who have been adequately treated with the 1- or 3-month versions of paliperidone palmitate, Janssen announced.
- OPM released weather leave guidance today according to Govexec.
- Healthcare Dive reports that “Four out of six infections routinely tracked at U.S. hospitals rose significantly during the COVID-19 pandemic, according to a Centers for Disease Control and Prevention data analysis published in the journal of the Society for Healthcare Epidemiology of America on Thursday. From 2019 to 2020, major increases were observed in central-line associated bloodstream infections, catheter-associated urinary tract infections, ventilator-associated events and antibiotic resistant staph infections, according to the report.”