Weekend update
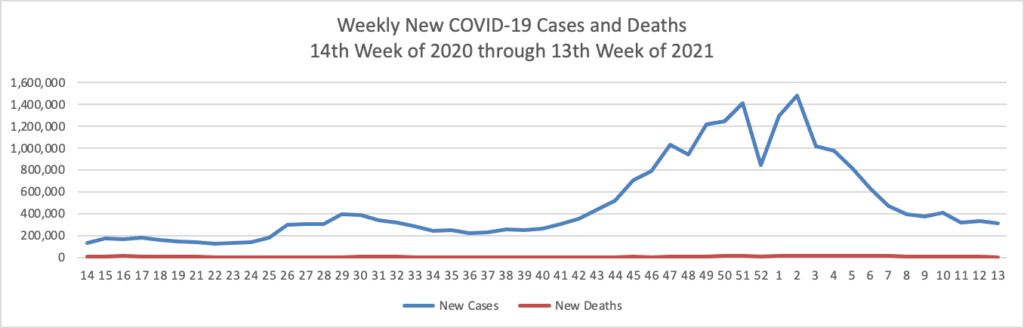
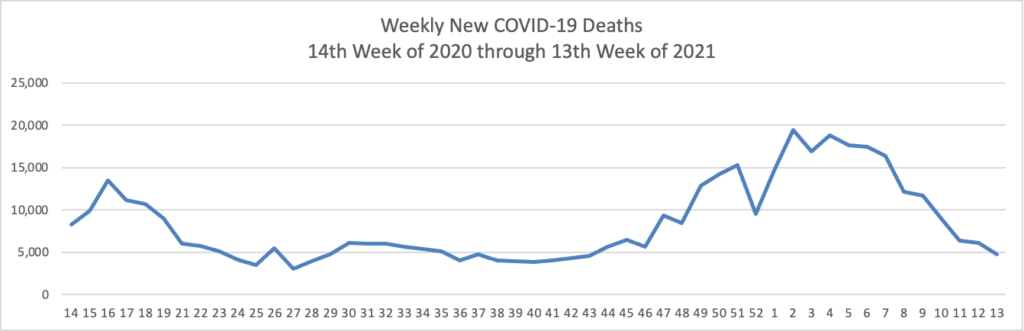
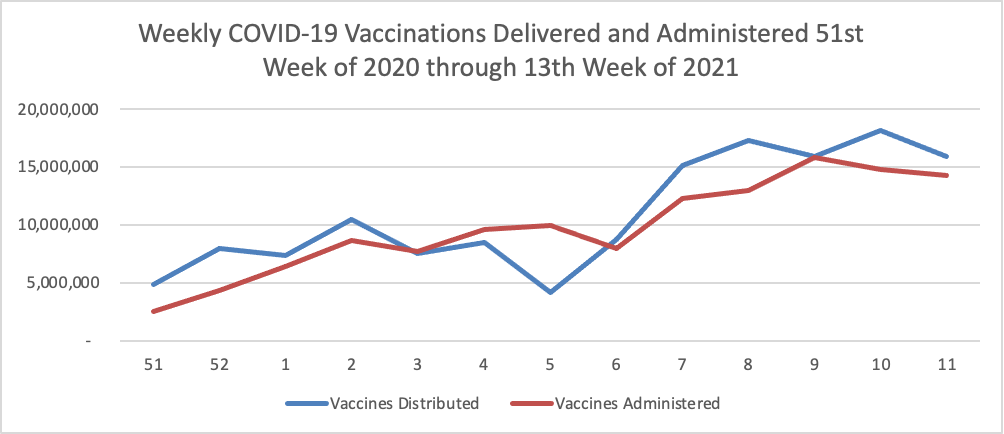
FEHBlog opening note — The FEHBlog goofed by posting this May 7 COVID-19 charts in the May 14 Friday Stats and More post. The FEHBlog corrected his error on Saturday after the Friday post email went out. You can check out the website if you want to see the May 14 charts which are encouraging. In contrast, check out the Wall Street Journal’s charts on the COVID-19 situation in India which is still struggling with virus. Whereas 37% of the U.S. population is fully vaccinated, less than 5% of the much larger and younger Indian population has reached that status. In this regard, the Rome (N.Y.) Sentinel offers an Excellus Blue Cross consulting pharmacist’s valuable guidance on why people in the age 18 to 34 bracket should received the COVID vaccination.
Q: Katie [Abbott, Pharm.D.], you are in that age group. Why did you choose to get vaccinated?
I trust the science behind the vaccines and believe they will help bring us back to how life was before the pandemic.
Q: Some, or most cases of COVID-19 in younger people are not severe. Why would a younger person get vaccinated if younger people aren’t really dying from COVID-19?
The younger population may not be seeing as many severe cases or deaths, but they are still at high risk of long COVID. Long COVID is when those who have recovered from COVID-19 experience lasting effects, including a range of symptoms such as fatigue, brain fog, chest pain, shortness of breath, cough, joint or muscle pain, depression, anxiety, and so much more. Long COVID can develop weeks or months after infection. It can happen to anyone who has had COVID, even if they had mild or no symptoms. Getting the vaccine remains a safe way to protect yourself, along with your community, family members, and those who cannot be vaccinated.
Returning to the regular weekend update, both Congress will be in session this week for Committee work and House and Senate floor votes. The House Oversight and Reform Committee will hold its third recent hearing on prescription drug costs on Tuesday morning. It’s worth noting that although the House Oversight and Reform Committee approved the Postal Reform bill (HR 3076) last week, the House Energy and Commerce and Ways and Means Committees also have jurisdiction over the bill. So we don’t know right now, when the bill may reach the House floor.
In OPM news, the Federal Times reports that
In anticipation of more employees returning to the office and in the spirit of May’s Mental Health Awareness Month, the Office of Personnel Management issued a tip sheet for agency human resource staff to better support employees at a vulnerable time. * * * In addition to communicating with employees about the usual resources available to them – such as the Employee Assistance Program and mental health treatments offered through Federal Employee Health Benefit plans – OPM encouraged agency work-life coordinators and HR professionals to be as communicative as possible about office safety procedures and available work schedule adjustments to ease any potential employee anxiety.
In other healthcare news,
- mHealth Intelligence discusses the work of University of West Virginia researchers who are seeking to determine the best mix of in-person and virtual care. “With telehealth use skyrocketing over the past year and a half due to the coronavirus pandemic, some have wondered if there’s a limit to its effectiveness. Is there a certain number of virtual visits that a patient – especially one with a chronic condition – should get, after which the technology outlasts its value? The answer, according the researchers at the University of West Virginia, is … uncertain.” While that outcome is surprising to the FEHBlog, the researchers have gone back to the drawing board.
- Fierce Healthcare reports that “GoodRx, a telehealth and drug-pricing comparison software company, acquired competitor RxSaver for $50 million in cash. The company closed the deal in late April, GoodRx reported during its first-quarter 2021 earnings call Thursday. RxSaver, which was owned by Vericast Corp., the payment and marketing company controlled by billionaire Ronald Perelman, operates a price comparison platform to provide discount offerings through partnerships with pharmacy benefit managers (PBMs). The acquisition will expand GoodRx’s business capabilities and consumer reach, particularly with respect to its prescription offering, the company said in its first-quarter 2021 earnings report.”
- Health Payer Intelligence informs us that ” To help combat racial care disparities in communities of color, Blue Shield of California (Blue Shield) provided $300,000 to 12 different nonprofit organizations in California that promote the mental health and well-being of youths in their communities. This act supports the health equity strategy of Blue Cross Blue Shield Association (BCBSA), Blue Shield’s parent company, as it seeks to improve racial care disparities by collaborating with local community leaders. By contributing $25,000 to each organization, Blue Shield is providing opportunities for youths of color that can improve their mental health.”
- Healthcare Dive reports that “Piedmont Healthcare signed a non-binding letter of intent to acquire Augusta, Georgia-based University Health Care System, which operates three hospitals as well as skilled nursing facilities and urgent care clinics along Georgia’s eastern border with South Carolina. * * * Just last week, the 11-hospital system announced plans to buy four additional hospitals from HCA Healthcare for $950 million. The sale is expected to close in the third quarter of this year. The hospitals in the HCA deal circle the outskirts of the Atlanta region. * * * Altogether, the two most recent deals would give Piedmont a total of 18 hospitals in Georgia, in addition to more ancillary services.” Healthcare Dive adds that the two deals are likely to face regulatory scrutiny.