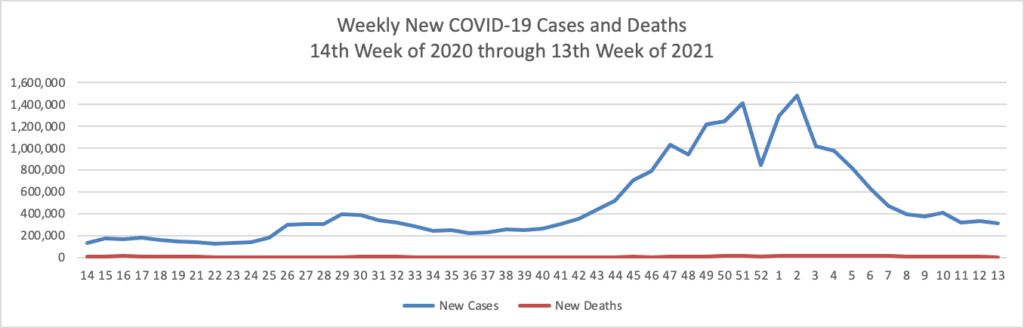
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 13th week of this year (beginning April 2, 2020, and ending March 31, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases greatly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the one year period (April 2, 2020 through March 31, 2021):
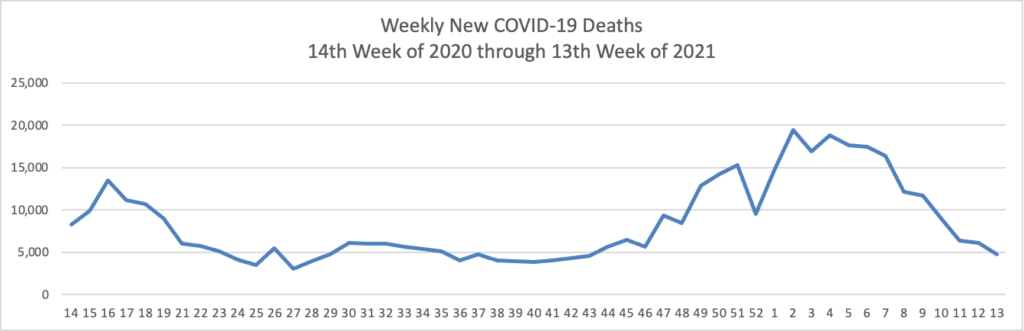
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through March 31, 2021, which also uses Thursday as the first day of the week:
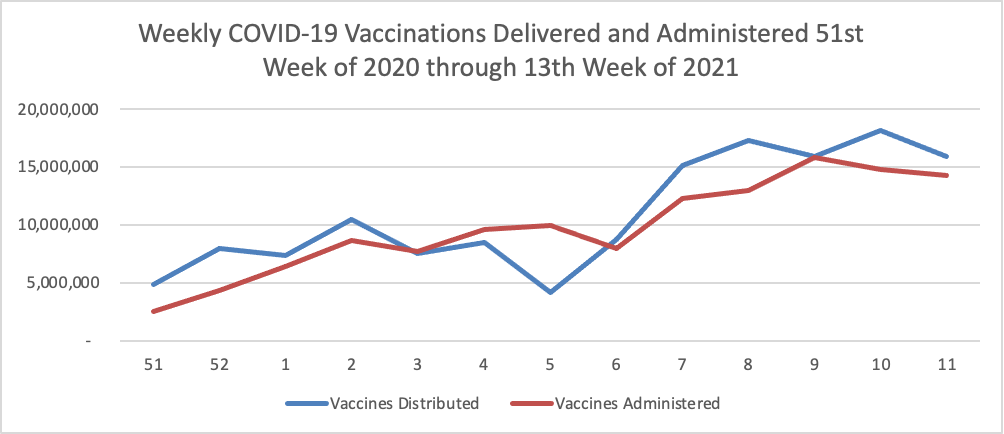
Bloomberg reported this afternoon that
More than 101 million people in the U.S. have received a first dose of a Covid-19 vaccine, or about 31% of the population.
Less than four months into the U.S. vaccination effort, coverage is best in people 65 years or older, with 74% of the group getting at least one dose and 54% completing vaccination, according to figures published by the Centers for Disease Control and Prevention and compiled by the Bloomberg Vaccine Tracker.
On Friday, the U.S. set a one-day record for vaccine doses reported administered, adding almost 4 million. On average, the U.S. is administering close to 3 million shots a day as of Friday’s update, also a record.
In other COVID-19 vaccination news
- Fierce Pharm reports that Johnson & Johnson which offers a one dose vaccine has joined the two dose vaccine manufacturers Pfizer and Moderna in its vaccine on adolescents aged 12-17.
- The Society for Human Resource Management informs us that
The following measures may increase vaccine acceptance in the workforce, according to the CDC:
1. Train interested staff to become COVID-19 vaccination ambassadors who will speak confidently and honestly, relaying personal stories about the vaccine to fellow co-workers and addressing any of their concerns.
2. Employ all available communication tools when promoting the COVID-19 vaccine to staff, including social media, internal communication channels, and posters or signs around the workplace.
3. Hold a virtual town hall where leadership, respected local medical experts and staff share their COVID-19 vaccine experiences and other vaccine facts and answer audience questions. Use experts to communicate to staff when talking about the COVID-19 vaccine. Ensure the experts present facts about the vaccine, including the risks.
4. Consider giving employees paid time off to get the vaccine and offering paid sick leave for employees who have adverse reactions.
5. Have workplace leadership take the COVID-19 vaccine, capture their experience using video or photo, and share the experience with staff.
- In this regard, Govexec.com reports that
Next week, the Health and Human Services Department plans to open a vaccination site for federal employees in the National Capital Region.
A source familiar with the plans told Government Executive on Friday that the site will be in Gaithersburg, Maryland. “The site will provide COVID vaccinations to federal, essential, critical infrastructure workers,” said the source. Federal agencies will determine eligibility based on “job duties and [the Cybersecurity and Infrastructure Security Agency’s] guidance on the essential, critical infrastructure workforce,” which includes 24 agencies.
The source was unsure at the moment on how many vaccine doses will be available or how many employees will be able to receive them. Employees will be notified starting Friday, April 2, the source said.
In health benefits news —
- The Labor Department’s Employee Benefits Security Administration released ACA FAQ 45 today. FAQ 45 provides compliance guidance on Section 203 of Division BB of the Consolidated Appropriates Act 2021. Section 203 requires health plans, including FEHB plans, to prepare and keep current written analyses demonstrating compliance with the non-quantitative treatment limitations requirements of the federal mental health parity law and implementing regulations.
- Fierce Healthcare explains how Optumcare successfully expanded the use of at home colon cancer screenings during the pandemic.
OptumCare used data analytics to flag the patients at risk for colon cancer and then reached out to them about the home test kits. While the kits included an information letter to describe the process, the clinical team also followed up four times by phone to check in with them.
If the testing results were positive or abnormal, the patient’s physician or care team would reach out directly to explain what the results meant and schedule them for future appointments to ensure care was coordinated throughout the process.
Frank said OptumCare saw a 5% higher return rate under the expanded program than in previous years, and the increased engagement drove interest in expanding other home health options such as home testing for blood glucose among diabetic patients.
- Fierce Healthcare also informs us that
The American Medical Association innovation subsidiary Health2047 has spun off a company that uses personalized medicine to fight obesity. Phenomix Sciences is a phenotype testing company that carries out the AMA’s mission to confront chronic diseases such as obesity.
Phenomix uses a blood test called MyPhenome that it has licensed from the Mayo Clinic to allow doctors to prescribe individualized therapies. MyPhenome measures DNA as well as a person’s metabolites and hormones. These biomarkers make up a person’s phenotype, according to Phenomix.
The company’s blood-based test uses phenotype-driven multi-omics technology to predict responses to obesity interventions that the Food and Drug Administration (FDA) has approved. A multi-omics test is important because testing for obesity involves multiple factors, including genetics, metabolomics and environmental aspects, according to Phenomix CEO Mark Bagnall.
Because patients respond differently to obesity treatment, the Phenomix founders turned to AI to personalize this treatment. AI can personalize a multi-omics obesity test and analyze single-nucleotide polymorphisms, metabolites and hormones that correspond with a certain obesity phenotype, according to Bagnall.
AI can help identify a specific obesity phenotype so patients can receive the right treatment.
“[We] demonstrated in several clinical studies that knowing a patient’s phenotype doubles the likelihood of weight loss and doubles the amount of weight lost,” Bagnall said.
