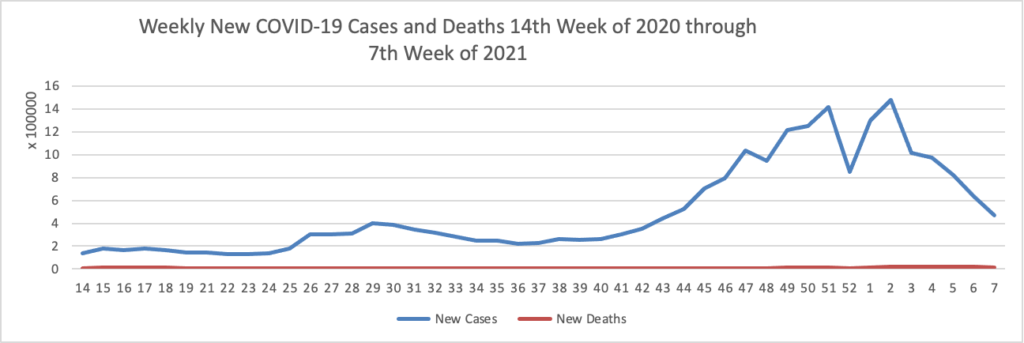
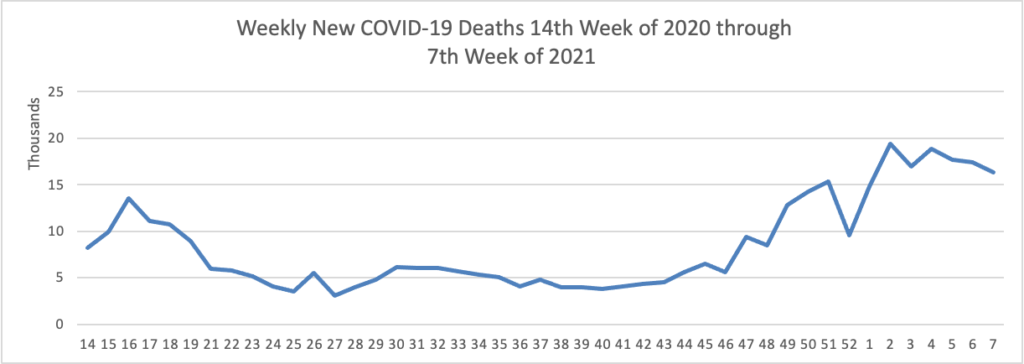
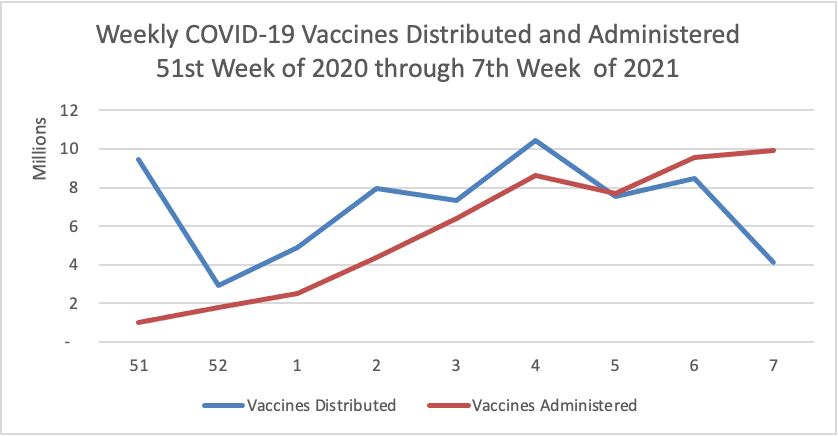
Thursday Miscellany

Roll Call reports that the Senate continues to move forward its modified version of the American Rescue Plan which the House of Representatives passed last week. Here’s a link to the Congressional Budget Office’s report on the Senate bill.
The Senate Homeland Security and Governmental Affairs Committee held a confirmation hearing today on the President’s nominations of Shalanda D. Young to be Deputy Director, Office of Management and Budget, and Jason S. Miller to be Deputy Director for Management, Office of Management and Budget. Federal News Network sums up the hearing as follows: “President Joe Biden’s picks to serve in top positions at the Office of Management and Budget vowed on Thursday to remove hurdles from federal hiring, improve employee morale and help agencies keep their workforces safe during the pandemic.”
David Leonhardt of the New York Times does a great job putting the three current COVID-19 vaccines in perspective:
It’s the latest case of vaccine alarmism.
Many Americans are worried that Johnson & Johnson’s Covid-19 vaccine is an inferior product that may not be worth getting. Gov. Doug Burgum of North Dakota recently told The Washington Postthat he was now seeing not only “vaccine hesitancy” but also “the potential for brand hesitancy.”
The perception stems from the headline rates of effectiveness of the three vaccines: 72 percent for Johnson & Johnson, compared with 94 percent for Moderna and 95 percent for Pfizer. But those headline rates can be misleading in a few ways.
The most important measure — whether the vaccine prevents serious illness — shows the Johnson & Johnson vaccine to be equally effective as the other two. All work for nearly 100 percent of people. The picture is murkier for mild cases, but they are not particularly worrisome.
In promising news, STAT News reports that
Eli Lilly said Thursday that a study showed its experimental diabetes drug, tirzepatide, reduced patients’ blood sugar and body weight more than a rival medicine, Novo Nordisk’s Ozempic. The study compared three doses of tirzepatide — 5 mg, 10 mg, and 15 mg — to a 1 mg dose of Ozempic. Both drugs were given as injections. Tirzepatide reduced A1C, a measure of blood sugar levels, by 2.09% at the 5-mg dose, 2.37% at the 10-mg dose, and 2.46% at the 15-mg dose. For Ozempic, there was a 1.86% reduction. Patients were followed for 40 weeks.
Patients who received tirzepatide also saw their body weight decline by more than those who received Ozempic. They lost an average of 7.8 kilograms, or 8.5% of their body weight at the lowest dose, 10.3 kg, an 11% decrease, on the middle dose, and 12.4 kg, a 13.1% decrease, on the highest dose. For patients on semaglutide, the decrease in body weight was 6.2 kilograms, or 6.7%.
The differences were all statistically significant.
Lilly plans to virtually present the full trial results at the American Diabetes Association’s annual scientific conference in late June 2021.
In concerning news, the Centers for Disease Control informs us that “a new paper from CDC, in partnership with the University of Utah, estimates that the national healthcare costs associated with infections from six multidrug-resistant pathogens can be substantial at more than $4.6 billion annually. This is one of the largest studies to estimate the cost associated with high-priority antibiotic-resistant pathogens. Issues highlighted in the study align with data and threats in CDC’s 2019 Antibiotic Resistance (AR) Threats Report. This includes the impact of resistant infections in the community, which can put more people at risk, make spread more difficult to identify and contain, and threaten the progress made to protect patients in healthcare.”
Finally, Fierce Healthcare lets us know that
Greater liquidity, a stable payer mix and higher-acuity patients helped major hospital chains end 2020 with massive profits despite a financial roller coaster caused by the pandemic.
The latest earnings reports from several for-profit and not-for-profit hospital chains come as patient volumes continue to drift below pre-pandemic levels and as major hospital groups have raised the alarm about financial hardship faced by many hospitals around the country.
And while plenty of health systems around the country are struggling, experts say many of the largest health systems around the nation have remained profitable.
[However] Rural and more independent and smaller facilities already operate on narrower profit margins which have been exacerbated by the pandemic. These financial headwinds could cause more consolidation among such facilities.