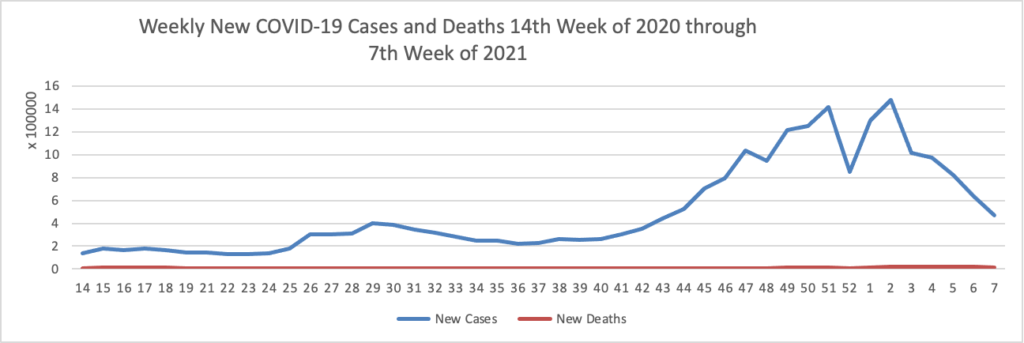
Based on the Centers for Disease Control’s COVID-19 Case Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through the 7th week of this year (beginning April 2, 2020 and ending February 17, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases greatly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths, which is a lagging indicator, over the period (April 2, 2020 through February 17, 2021):
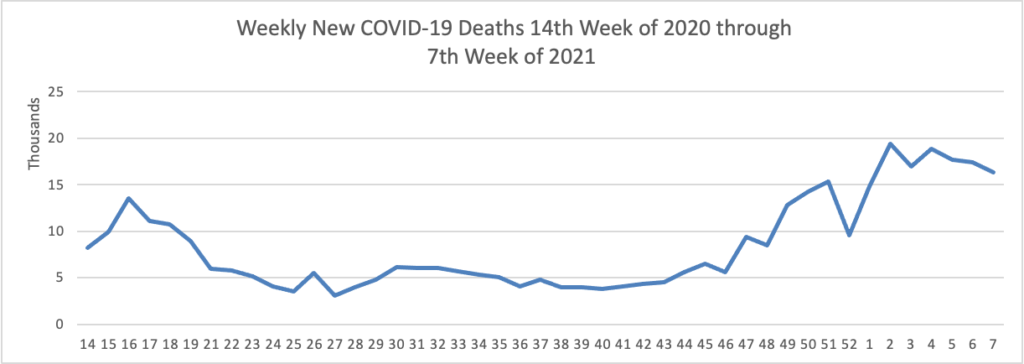
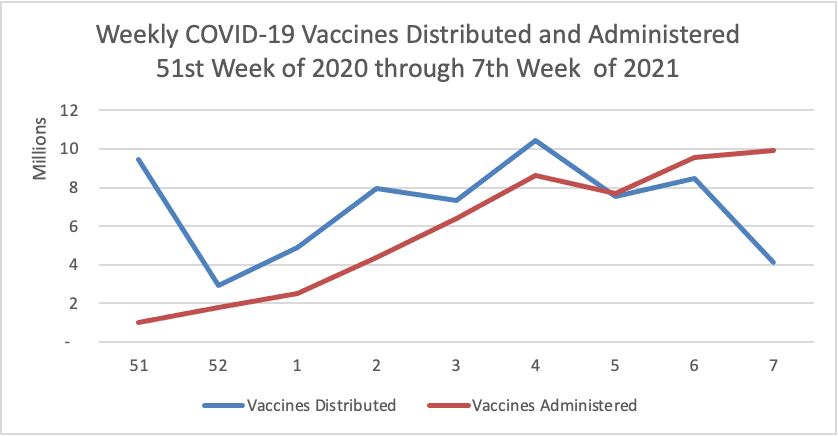
Finally here is a COVID-19 vaccinations chart for the past month which also uses Thursday as the first day of the week:
The Wall Street Journal reports tonight that
Efforts to vaccinate the world’s population against Covid-19 got a boost Friday after research showed that some vaccines provide strong, one-dose protection, and that one of the vaccines can now be stored in normal freezers instead of ultra-cold ones.
The vaccine developed by Pfizer Inc. and BioNTech SE generates robust immunity after one dose, according to new research out of Israel, and further data showed that the University of Oxford and AstraZeneca PLC vaccine similarly prevented Covid-19 when doses were spaced three months apart.
The findings could boost arguments in favor of delaying the second dose of the two-shot vaccine, as the U.K. has done. They could also have substantial implications on vaccine policy and distribution around the world, simplifying the logistics of distribution.
Pfizer and BioNTech said they have asked U.S. regulators to allow their vaccine to be stored and transported at temperatures consistent with standard freezing, around minus 20 Celsius, following successful internal stability testing. Similar filings were being prepared in other countries.
Should Pfizer’s request be granted by regulators, it would mean its vaccine would vastly expand access in rural regions around the world, as well as pharmacies and physician offices, according to industry experts and officials.
The New York Times has a great article on combatting COVID-19 alarmism and the Society for Human Resource Management discusses the uncertain legal state of employer offers of COVID-19 vaccination incentives to their employees in an effort to overcome vaccine reluctance.
In federal personnel news –
- OPM announced to FEHB carriers today the promotion of Laurie Bodenheimer to Associate Director, Healthcare and Insurance. Ms. Bodenheimer has served as acting Director of Healthcare and Insurance for the past two and half years. The FEHBlog notes that under federal law, 5 U.S.C. § 1102(d)
There may be within the Office of Personnel Management not more than 5 Associate Directors, as determined from time to time by the Director. Each Associate Director shall be appointed by the Director.
So congratulations Laurie for your well deserved appointment.
- Fierce Healthcare reports that “President Joe Biden has chosen Obama administration veteran Liz Fowler to lead the Center for Medicare and Medicaid Innovation (CMMI), which has authority to shape key payment models, according to a report in Politico.” This powerful position does not require Senate confirmation.
