Tuesday Tidbits

Fortune offers an insightful story about CVS Health’s CEO Karen Lynch.
Smart Brief discusses news from last week’s AHIP National Policy Conference.
The Society for Human Resource Management offers a helpful list of American Rescue Plan Act provisions impacting employers.
Medscape encouragingly reports that
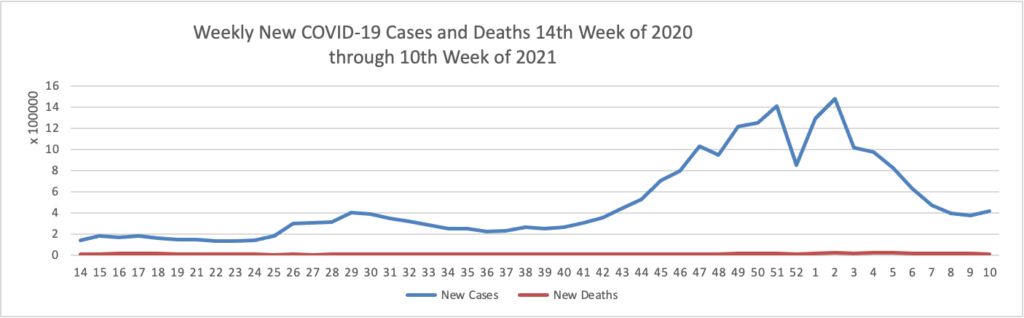
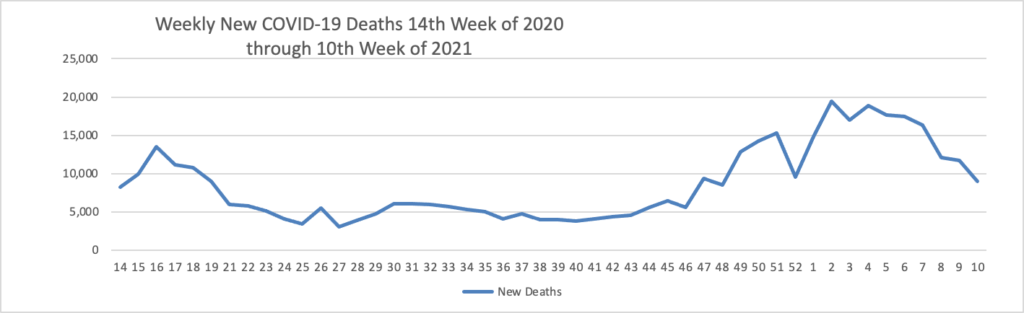
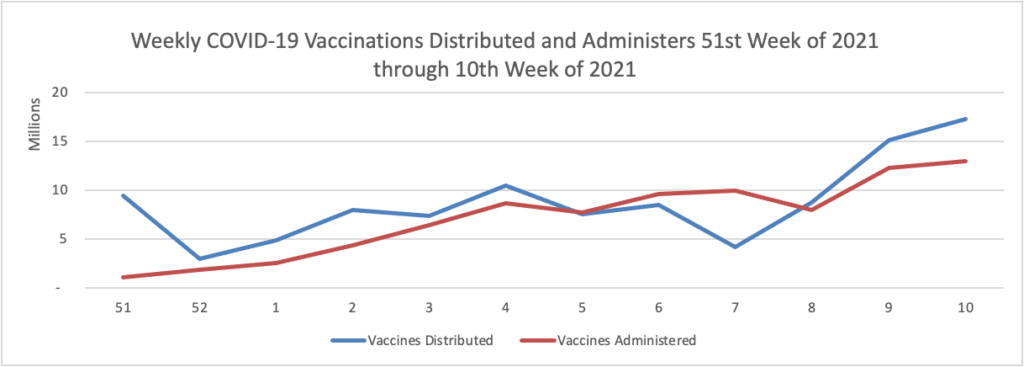
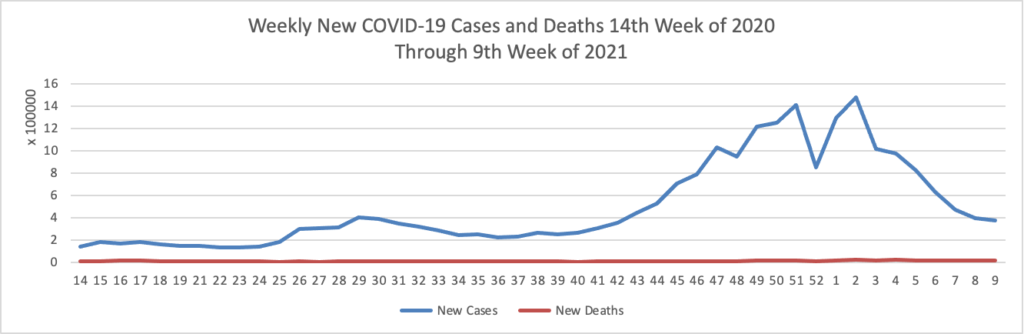
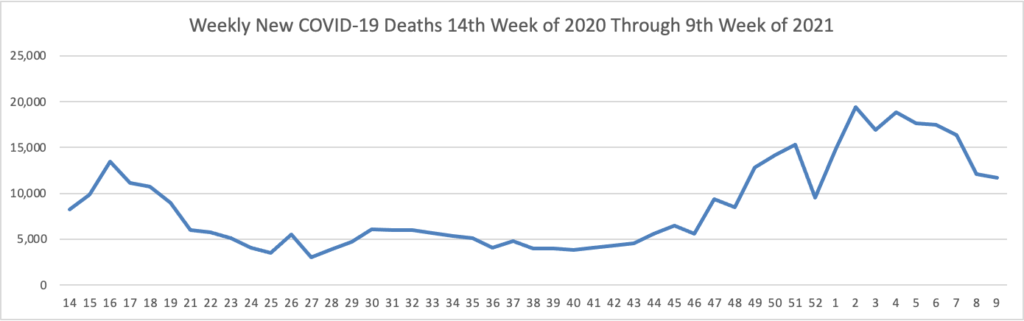
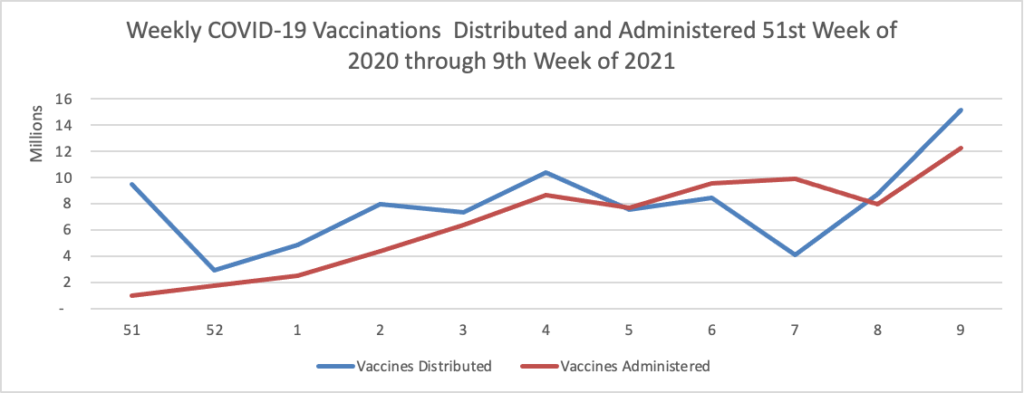
Vaccination of about 88% of Americans who received the first dose of Pfizer/BioNTech or Moderna’s COVID-19 vaccines was complete, a study of over 12 million people by the U.S. Centers for Disease Control and Prevention (CDC) showed. * * * According to the analysis, about 3% of people in the United States who received the first dose of either vaccine did not get the second dose needed to complete vaccination. The agency said 8.6% had not received the second dose, but were still within the allowable interval to receive it.
As of today, 64.6% of the U.S. population over age 65 has received at least one dose of the COVID-19 vaccine and 36.6% of that group (including the FEHBlog) are fully vaccinated.
Healthcare Dive informs us that “Independent primary care docs more financially stable, but fed up with vaccine exclusion.” The FEHBlog heard today that vaccine distribution will open to more sites of care, including physician offices, once the Food and Drug Administration gives full marketing approval to the COVID-19 vaccines. The FEHBlog, however, could not find a projected date for that action, but he will keep looking.
Healthcare Dive also reports that “Virtual care company Doctor on Demand and clinical navigator Grand Rounds have announced plans to merge, creating a multibillion-dollar digital health firm.” The companies’ joint press release explains
The new company will combine Grand Rounds’ data-driven clinical navigation platform and patient advocacy tools with Doctor On Demand’s preeminent virtual care offering to provide an unparalleled member experience. It will accelerate the adoption of virtual care in key areas including primary care, specialty care, chronic condition management, and behavioral health. Owen Tripp, CEO of Grand Rounds, will serve as the CEO of the expanded business. Both companies will continue to operate under their existing brands for the time being.
“No one has done this before, combining navigation and virtual care delivery. We think it’s the future,” said Owen Tripp, co-founder and CEO of Grand Rounds. “People make unguided healthcare decisions every day, often with higher costs and worse outcomes. Now, with Doctor On Demand, we’ll offer them coordinated support on all fronts—physical, behavioral, financial, administrative—and we’ll do it for everything from acute issues to life-long health. This is truly complete care, and it’s what we all need.”
“We’re building a next-generation virtual care company with a nationwide practice of diverse, dedicated providers and a multidisciplinary care team,” said Hill Ferguson, CEO of Doctor On Demand. “By fully integrating medical and behavioral healthcare with clinical navigation, we’re impacting healthcare where it actually happens—between a patient and their provider—and ensuring that experience is seamless, personalized, and can follow the patient wherever they go.”
In continuing recognition of Patient Safety Awareness Week, here are links to the Agency for Healthcare Research and Quality’s blog post on accelerating progress in patient safety and an AHRQ article on the importance of good communication skills to achieving patient safety.