Tuesday’s Tidbits

The Wall Street Journal reports
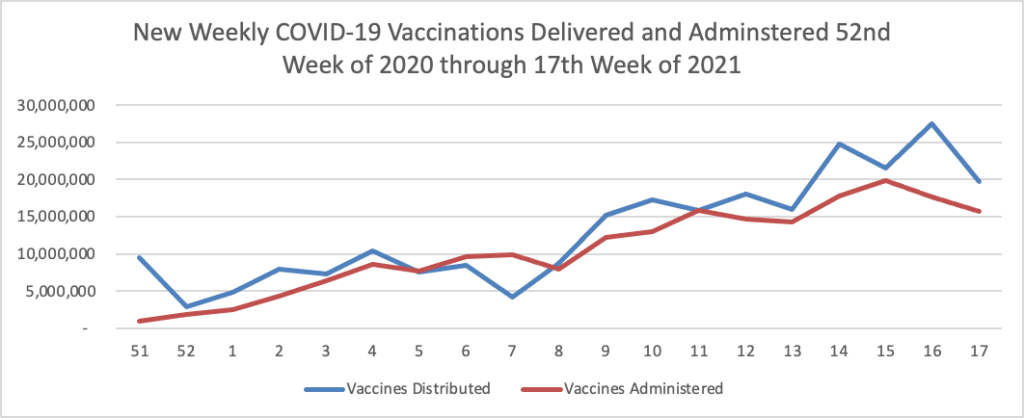
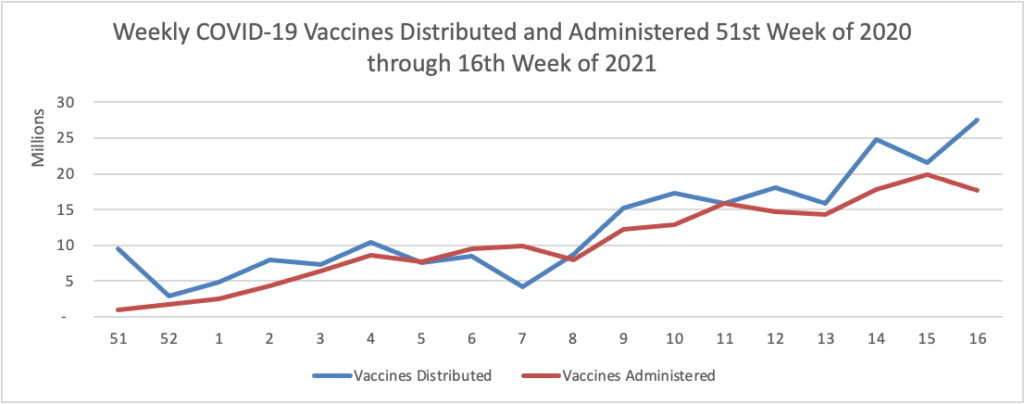
The Biden administration said it would begin reallocating some Covid-19 vaccine doses to states with higher demand for shots and direct pharmacies to offer walk-in vaccinations, as the president aims to get 70% of the adult population at least one dose by July 4.
President Biden said Tuesday he also wants 160 million U.S. adults to have the full course of the vaccine by that point, which he said would mean administering about 100 million shots over the next 60 days. The U.S. administered about 220 million shots in Mr. Biden’s first 100 days, but the pace of vaccinations has fallen in recent weeks, according to the Centers for Disease Control and Prevention. Roughly 56% of U.S. adults had received at least one dose as of Monday, according to the CDC.
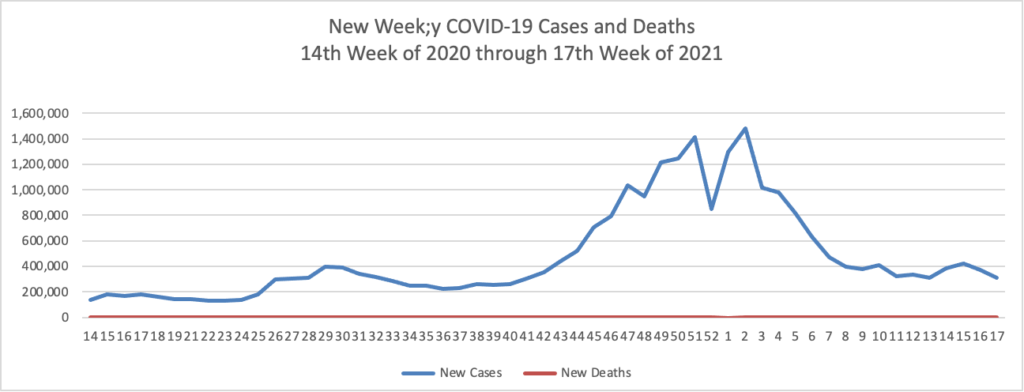
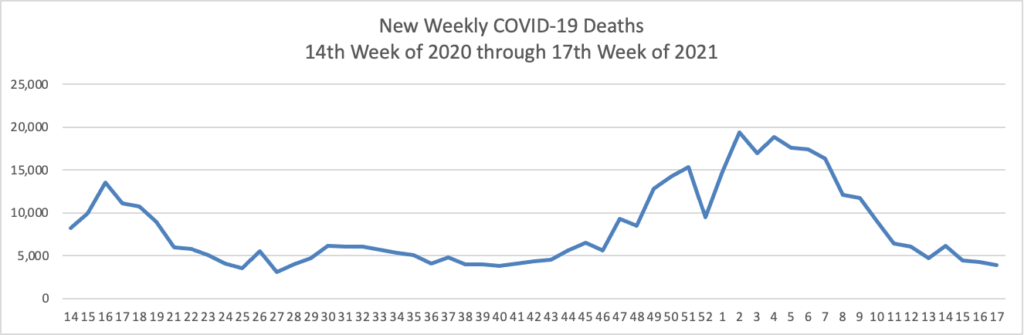
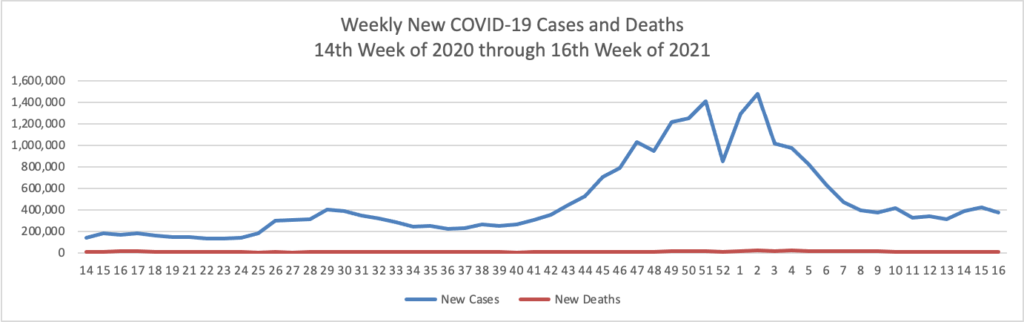
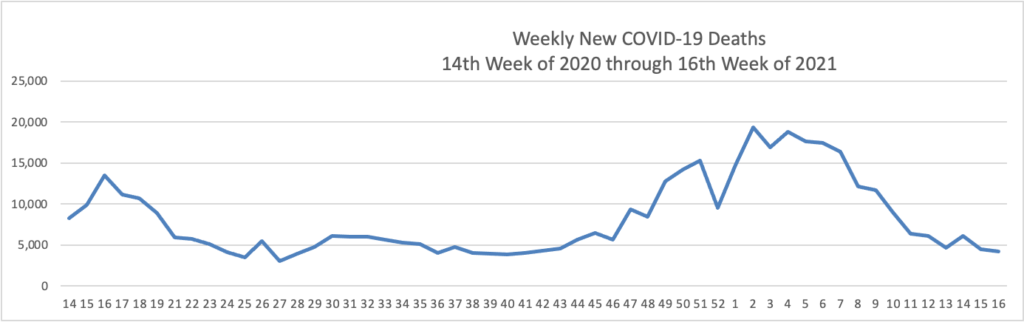
Also from the COVID-19 front, the NIH Director Dr. Francis Collins offers a real world look at COVID-19 vaccines versus variants.
Healthcare Dive reports
- CVS Health beat Wall Street expectations for earnings and revenue in the first quarter, reporting a topline of $69.1 billion, up 3.5% year over year due to growth across all major businesses.
- The diversified healthcare behemoth brought in net income of $2.2 billion, compared to $2 billion at the same time last year in financial results released premarket Tuesday.
- Following the quarter, which saw a strong financial showing from all major U.S. payers, CVS raised its full-year earnings guidance, noting it expects normal utilization throughout 2021 and minimal effects from the COVID-19 pandemic. However, management did warn vaccine hesitancy could slightly hamper expected earnings growth.
In related CVS news —
- NPR Shots informs us about how CVS Health is adding mental health therapists to its Minute Clinics and Health Hubs.
- Drug Channels places CVS Specialty at the top of its list of top 15 specialty pharmacies.
In other healthcare business news
- STAT News informs us that “Although the pharmaceutical industry argues that wholesale prices do not accurately reflect prescription drug costs, a new study finds that rising wholesale prices have, in fact, led to higher out-of-pocket expenses for roughly half of insured patients.” Shocking.
- The Wall Street Journal reports that “Pfizer Inc. raised this year’s sales forecast for its Covid-19 vaccine to about $26 billion, a 73% increase that reflects the shot’s growing role in a long-term global vaccination campaign.” Thanks Pfizer.
- Health Payer Intelligence reports
Gross margins and medical loss ratios from 2020 may confirm that payer profitability increased during the coronavirus pandemic, according to a brief from Kaiser Family Foundation.
The researchers leveraged data from the National Association of Insurance Commissioners (NAIC) to observe the pandemic’s effects on the profitability of four health insurance markets: Medicare Advantage, Medicaid managed care, the individual health insurance marketplace, and the fully-insured group health insurance marketplace.
“By the end of 2020, gross margins per member per month across these four markets remained relatively high and medical loss ratios were relatively low or flat compared to recent years,” the researchers discerned. “These findings suggest that many insurers remained profitable through 2020.”
Here are some additional healthcare tidbits —
- The Lown Institute announced that “Every 80 seconds, a hospital in the U.S. delivers a low-value test or procedure to an older adult, putting hundreds of thousands at risk of harm, according to a new analysis from the Lown Institute, a health care think tank. The Institute today released a ranking of over 3,100 U.S. hospitals that examines success at avoiding the use of tests and procedures that offer little to no clinical benefit.”
- The Congressional News Service released a helpful report titled “A Comparison of Tax-Advantaged Accounts for Health Care Expenses.”
- The Department of Health and Human Services announced “the availability of nearly $1 billion to strengthen COVID-19 response efforts and increase vaccinations in rural communities. As part of the Biden Administration’s commitment to expanding access to vaccines and ensuring equity in the COVID-19 response, the Health Resources and Services Administration, a part of HHS, will increase the number of vaccines sent to rural communities, expand testing and other COVID-19 prevention services, and work to increase vaccine confidence by empowering trusted local voices with additional funding for outreach efforts in underserved communities.”
Last but not least here are some federal employment tidbits —
- The Office of Personnel Management reminds us that “Each year Federal Executive Boards (FEBs) across the nation recognize federal employees who have made exceptional contributions in their community or the advancement of their agency’s mission. This year, OPM and the Partnership for Public Service are highlighting more than 300 awards winners from FY2020 and FY2021. To learn more about these recipients and their exemplary accomplishments, visit the FEB awards site. “
- Federal News Network reports that “Union leaders say staffing shortages are stretching their agencies thin.”
- Govexec reports that “Some U.S. Postal Service employees will receive layoff notifications later this month, the mailing agency told workers in a memorandum this week. * * * USPS declined to specify how many positions would be eliminated, but said it aims to offer impacted workers opportunities for reassignment.”