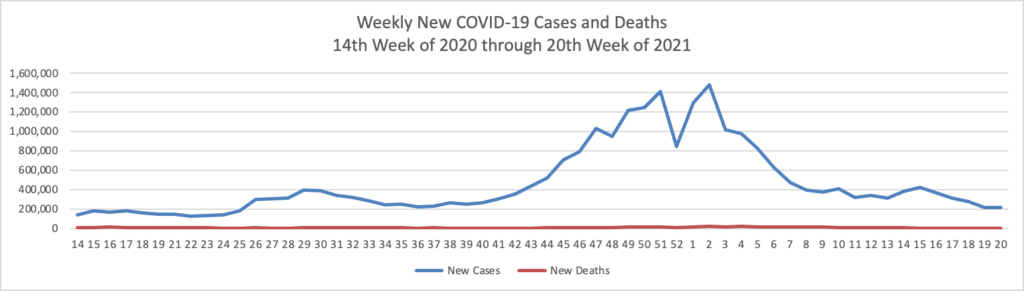
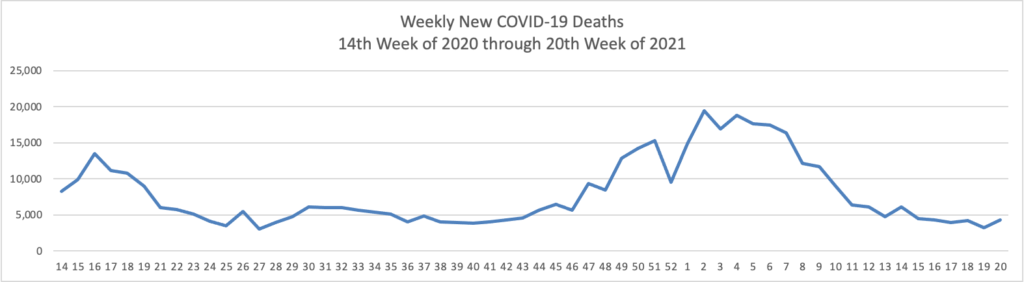
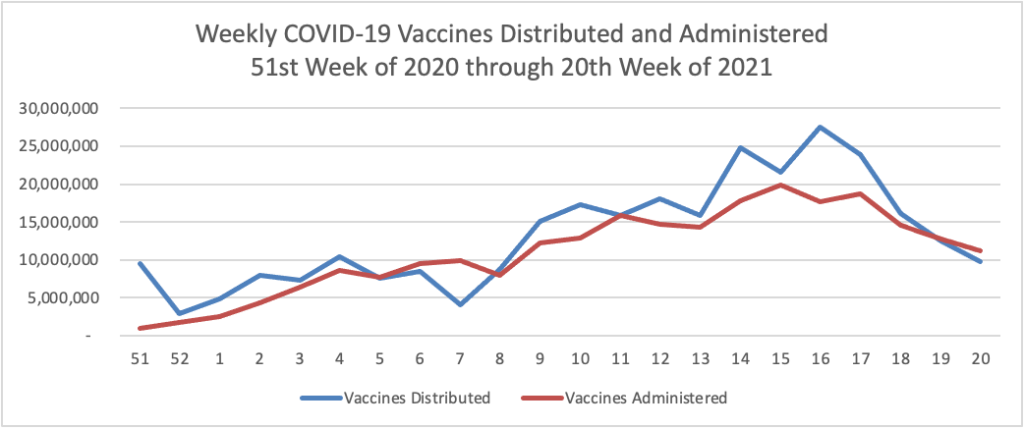
Memorial Day Update

On this second (and hopefully last) Memorial Day of the COVID-19 pandemic, let’s take a look at recent reports on the nation’s COVID-19 vaccination campaign —
- The Wall Street Journal reports that while vaccination rates in the Southeastern states have been relatively low, COVID-19 transmission has been slowed in those states by a combination of vaccinations supplemented by people engaging in open air activities.
Health officials in warm-weather states have launched public-information campaigns to encourage residents to spend as much time outside as possible and are pushing for more vaccinations at outdoor sites such as state parks and minor league baseball parks, as a way of heading off another potential summer surge this year.
“Vaccination and people spending time outdoors are the two biggest factors” in why cases have been relatively low this spring, said Brannon Traxler, an epidemiologist who heads South Carolina’s public-health division.
Encouraging people to spend time outside is a high priority, but the even bigger priority is the focus on vaccination, Dr. Traxler said: “If we can get folks vaccinated, then the weather and where and how people congregate becomes less of a problem.”
- The Centers for Disease Control reports on Patterns in COVID-19 Vaccination Coverage, by Social Vulnerability and Urbanicity from December 2020 through April 2021:
What is already known about this topic?
Counties with higher levels of social vulnerability have been disproportionately affected by COVID-19.
What is added by this report?
Disparities in county-level vaccination coverage by social vulnerability have increased as vaccine eligibility has expanded, especially in large fringe metropolitan (areas surrounding large cities, e.g., suburban) and nonmetropolitan counties. By May 1, 2021, vaccination coverage among adults was lower among those living in counties with lower socioeconomic status and with higher percentages of households with children, single parents, and persons with disabilities.
What are the implications for public health practice?
Outreach efforts, including expanding public health messaging tailored to local populations and increasing vaccination access, could help increase vaccination coverage in counties with high social vulnerability.
- The San Francisco Chronicle reports that “Hope builds that COVID vaccine boosters won’t be needed for a year – or much long.” “’I expect we will have to be revaccinated eventually, but I don’t think it’s going to be in a year-or-two time frame,’ said Dr. Joel Ernst, an infectious disease expert at UCSF. ‘Obviously we don’t have a long period of observation, because we haven’t had the vaccines that long. But so far I’m reassured that the vaccine-induced immunity seems to be pretty durable.’”
In other news
- Healthcare Dive informs us that “The Mayo Clinic reported turning a profit in the first quarter of the year, although its expenses rose significantly compared to the first quarter of 2020, which was mostly completed before the COVID-19 pandemic became a significant issue. * * * [M]ost patient volumes returned to pre-pandemic levels. Outpatient visits for the quarter matched 2019 numbers, while surgical volumes exceeded those of 2019, although inpatient days were down.”
- Kaiser Health News tells us that “The Biden administration said Friday it has no timeline on whether it will allow states to import drugs from Canada, an effort that was approved under President Donald Trump as a key strategy to control costs. Six states have passed laws to start such programs, and Florida, Colorado and New Mexico are the furthest along in plans to get federal approval. The Biden administration said states still have several hurdles to get through, including a review by the Food and Drug Administration, and such efforts may face pressures from the Canadian government, which has warned its drug industry not to do anything that could cause drug shortages in that country.” Smart move by the Administration. Our country’s population dwarfs Canada’s.
- The Wall Street Journal reports that “Genetically altered mosquitoes target deadly Dengue fever and ZikaIn pioneering test, insects with a gene primed to interrupt breeding are flying in the Florida Keys. Go get em. One pandemic is enough.