Friday Stats and More
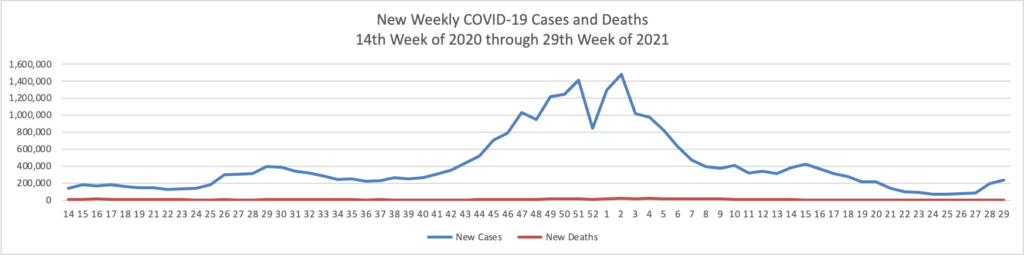
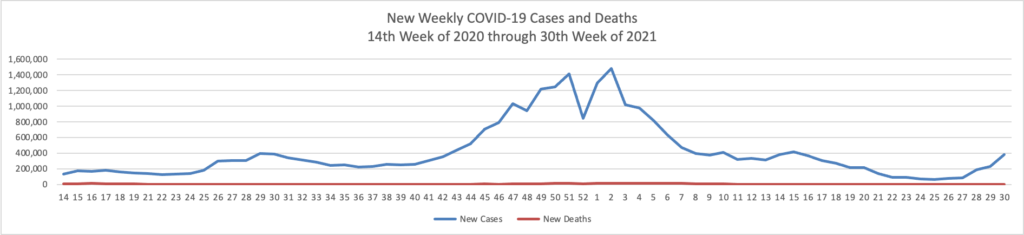
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 30th week of this year (beginning April 2, 2020, and ending July 28, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

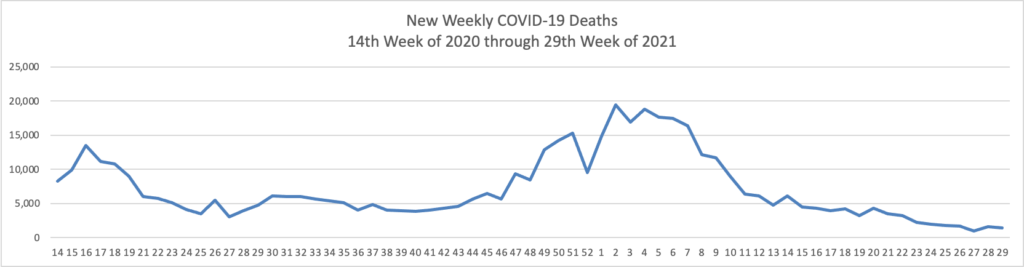
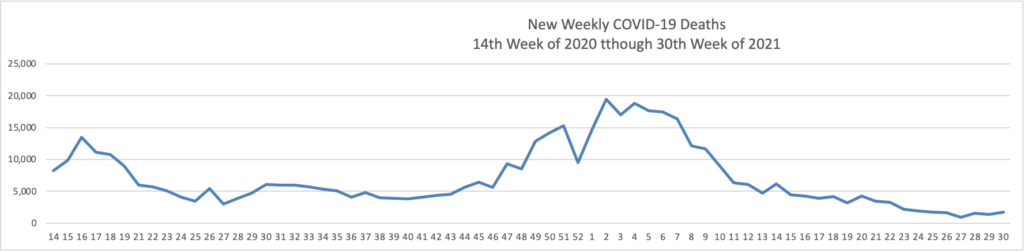
The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases significantly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through July 28, 2021):
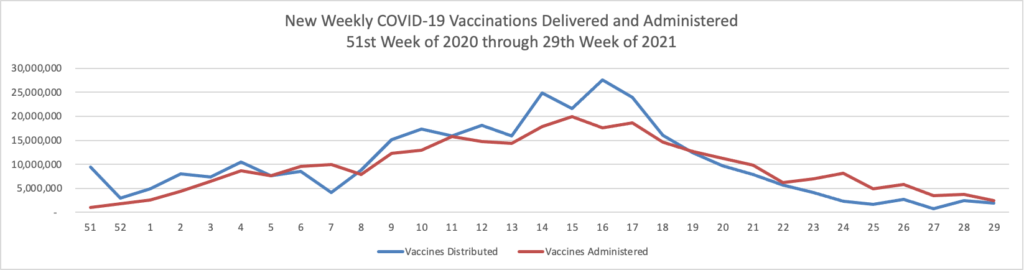
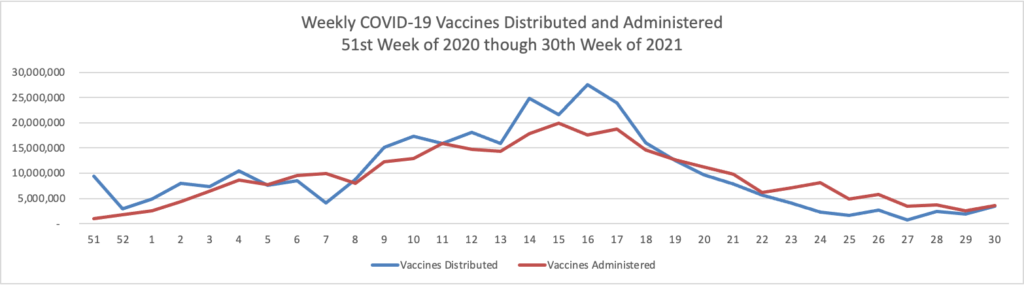
Finally here is a COVID-19 vaccinations chart over the period December 17, 2020, through July 28, 2021, which also uses Thursday as the first day of the week:
Due to the Delta variant, new cases and hospitalizations are trending up while new deaths have remained low for two months.
Happily COVID-19 vaccinations are trending up again. As of today according to the CDC, 60% of the U.S. population over age 18 and 80% of those over age 65 are fully vaccinated. GEHA, the largest employee organization plan in the FEHB, announced that it has extended its COVID-19 vaccine incentive program to Labor Day, September 6.
The CDC defended its new masking policy for the vaccinated population by pointing to a case in which as reported by the Wall Street Journal
127 vaccinated people infected with the Delta variant during the outbreak appeared to carry as much virus as 84 unvaccinated or partially vaccinated people who became infected. The report referred to an outbreak in Barnstable County, Mass. Local officials there have said that at least 430 confirmed Covid-19 cases have been linked to one cluster following festivities over the July 4 weekend in Provincetown, on the tip of Cape Cod.
Among the 469 cases linked to the Barnstable outbreak in the CDC report, nearly 75% were fully vaccinated. For people with breakthrough infections, almost 80% had symptoms of cough, headache, sore throat or fever. Four were hospitalized and no deaths were reported, the CDC said. Infected people reported attending densely packed indoor events and outdoor events at bars, restaurants and houses.
Toward the conclusion of the article the journalist speaks with Dr. Ashish Jha, dean of the public-health school at Brown University.
Dr. Jha said he thinks this week’s guidance recommending masking in high-risk areas of the U.S. was reasonable, but also risked suggesting that vaccines aren’t effective against the Delta variant, which could discourage unvaccinated people from getting shots. We have the tools to address this variant, and they’re called vaccines,” Dr. Jha said.
The FEHBlog certainly would wear a mask at an indoor or outdoor super spreader event in a high risk area like the one where the FEHBlog is temporarily living, Travis County Texas. It’s worth noting this US Health Weather map which gauges the risk of catching a respiratory infection like COVID-19 or the flu in a particular US county. Ironically, my county of permanent residence, Montgomery County, Maryland, is low risk.
STAT News adds that the Food and Drug Administration is accelerating the process of reviewing Pfizer-Biotech’s application for full marketing approval of their COVID-19 vaccine. (Moderna also has made this filing.)
A typical review of an application like Pfizer’s takes 10 months. The agency granted Pfizer a “priority review” for its vaccine earlier this month, which signifies that staff will strive to finish the review of the application within six months. At the same time, he FDA has said it does not expect the process to take that long — a view echoed even by President Biden.
“My expectation … is that sometime, maybe in the beginning of the school year, at the end of August, beginning of September, October, they’ll get a final approval” Biden said last week when asked when the FDA would formally approve the Covid-19 vaccines, including the one developed by Pfizer and its partner BioNTech.
Jesse Goodman, who led the FDA’s biologics center from 2003 to 2009, said that the August-September time frame is “possible … if all goes smoothly.” He said the idea of a sprint is “reasonable,” so long the biologics center follows the normal chain of command for reviewing these applications.
In other news
- Federal News Network tells us that “The House of Representatives on Thursday cleared a $600 billion package of seven spending bills, a small step forward in boosting civilian agency funding next year.The seven-bill “minibus” cleared the House Thursday afternoon by a 219 to 208 vote. The minibus is silent on federal pay for 2022, a silent endorsement of President Joe Biden’s proposed 2.7% raise for civilian employees. * * * The spending package also includes $42 million for the Office of Personnel Management over current levels and allows the agency to stand up an IT working capital fund.” The House is close to completing approval of the twelve appropriations bills. The Senate has not begun to vote on those bills and new federal fiscal year begins in two months, October 1.
- The Congressional Budget Office released its financial analysis of the President’s budget proposal for the upcoming new federal fiscal year.
- The ICD10 Monitor explains that yesterday the Centers for Medicare and Medicaid Services finalized four Medicare Part A payment rules which take effect on October 1 — skilled nursing facilities (SNFs), hospices,
- inpatient rehabilitation facilities (IRFs), and inpatient psychiatric facilities (IPFs). The Monitor’s article summarizes each final rule.
- The American Hospital Association offers a useful article on approaches to resolving COVID-19 vaccine hesitancy.
- Fierce Healthcare informs us about insurer comments on the third 2022 Notice of Benefit and Payment Parameters, which proposed changes to the ACA marketplace.