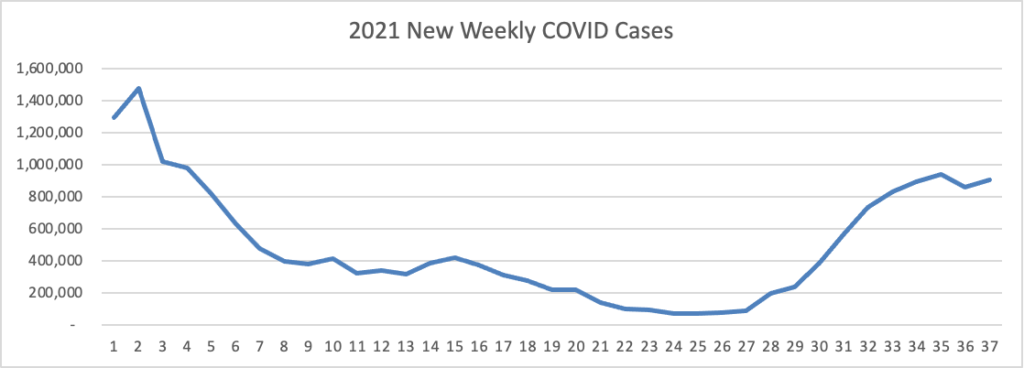
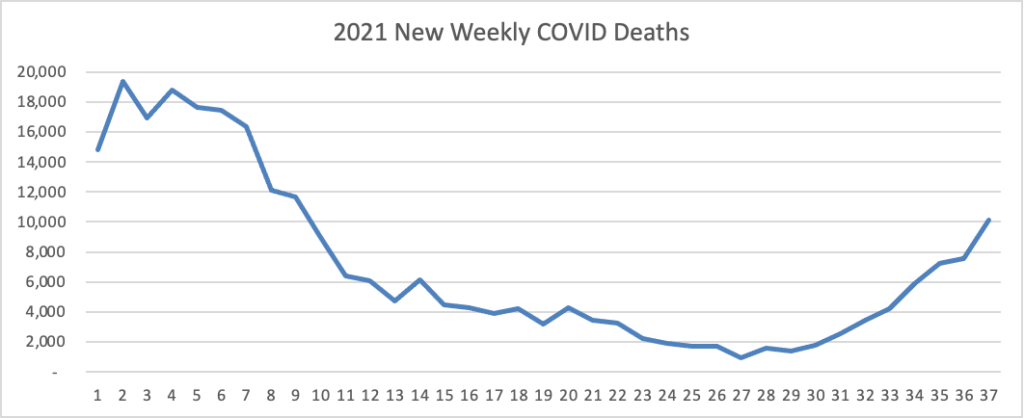
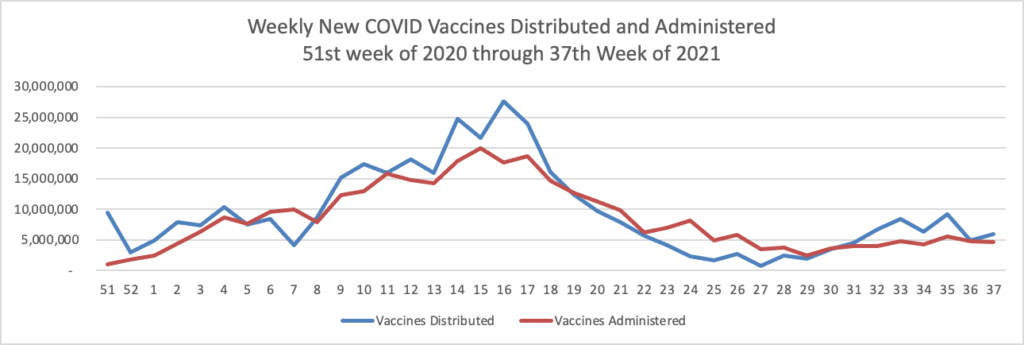
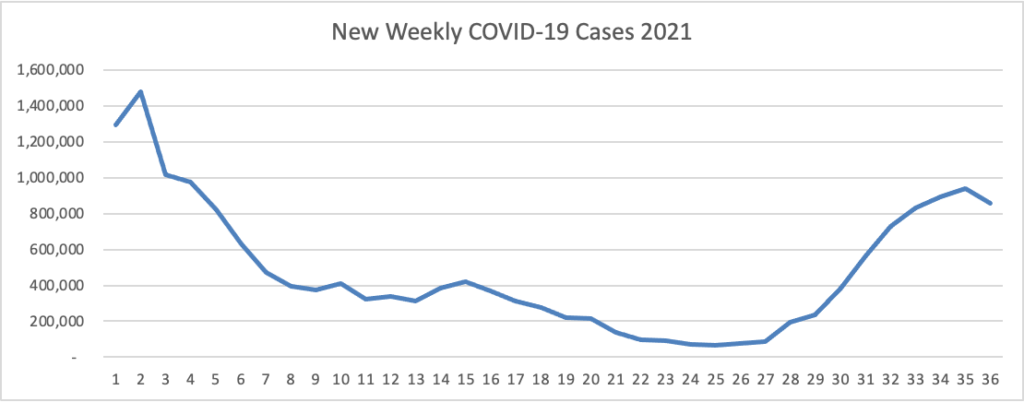
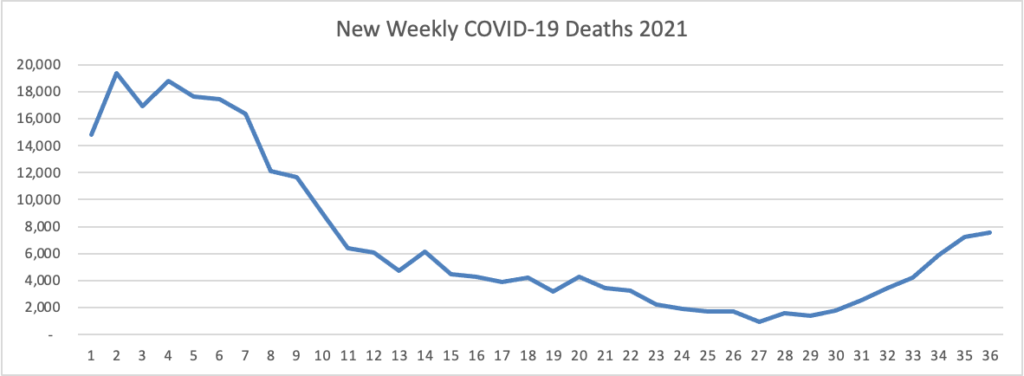
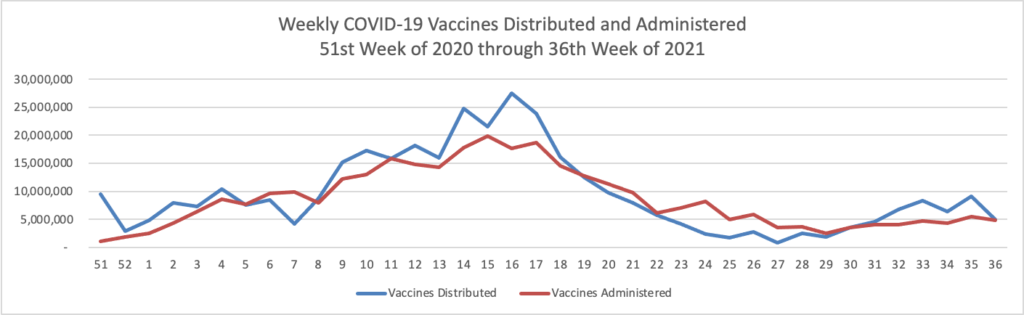
ACIP Ratifies FDA Approach to COVID-19 Boosters

In an audible triggered by yesterday’s decision, the CDC’s Advisory Committee on Immunization Practices considered at today’s meeting the FDA’s approach to COVID-19 boosters. AHIP informs us that
ACIP voted today to recommend a Pfizer-BioNTech COVID-19 vaccine booster dose for:
persons aged 65 and older and long-term care facility residents;
persons aged 50 to 64 with underlying medical conditions; and
persons based on individual benefit and risk who are aged 18 to 49 years with underlying medical conditions.
Following robust discussion about the risks and benefits of each option, ACIP ultimately voted to recommend a Pfizer vaccine booster dose, at least 6 months after the primary series, for persons aged 65 and older and long-term care facility residents (by a vote of 15-0), persons aged 50 to 64 with underlying medical conditions (13-2), and persons based on individual benefit and risk who are aged 18 to 49 years with underlying medical conditions (9-6).
The Committee voted 9-7 against recommending a single Pfizer-BioNTech COVID-19 vaccine booster dose based on individual benefit and risk for persons aged 18-64 years who are in an occupational or institutional setting where the burden of COVID-19 infection and risk of transmission are high based on safety concerns and patient selection.
ACIP only voted on boosters for individuals in each group who had previously received the initial series of the Pfizer vaccine; FDA indicated in its Emergency Use Authorization (EUA) that there was insufficient evidence to consider using a Pfizer booster in patients who received another vaccine in the initial series and thus it was not considered in the ACIP recommendations.
The next step in the health plan coverage process is for the CDC to ratify the ACIP decision. 15 days thereafter federal law requires health plans to cover the Pfizer-BioNTech booster in these scenarios with no member cost sharing in or out of network.
In additional news from that meeting, AHIP tells us that
ACIP also evaluated data on the COVID-19 vaccine in pregnant people and adverse outcomes associated with COVID-19 infection in this population. Data showed there is no indication that vaccines are associated with spontaneous abortion, birth defects, or stillbirths, but that pregnant people who were infected with COVID-19 exhibited increased risk of preeclampsia, preterm birth, NICU admission, and death.
In related news, Bloomberg reports that
Pregnant women who get mRNA vaccines pass high levels of antibodies to their babies, according to a study published in American Journal of Obstetrics & Gynecology – Maternal Fetal Medicine on Wednesday.
The study — one of the first to measure antibody levels in umbilical cord blood to distinguish whether immunity is from infection or vaccines — found that 36 newborns tested at birth all had antibodies to protect against Covid-19 after their mothers were vaccinated with shots from Pfizer Inc.–BioNTech SE or Moderna Inc.
“We didn’t anticipate that. We expected to see more variability,” said Ashley Roman, an obstetrician at NYU Langone Health System and co-author of the study.
That is certainly good news.
From the health equity front —
- The Government Accountability Office issued a health care capsule on this topic. GAO’s “2-page “capsule” draws from several GAO reports to provide examples of these health disparities, such as COVID-19, maternal mortality, chronic health conditions, as well as disparities among veterans. We also offer policy considerations to help the federal government better understand health disparities and promote health equity.”
Healthcare Dive reports that
Only 75 of the 3,000 hospitals ranked by the Lown Institute Hospitals Index scored top marks across all the metrics meant to evaluate social responsibility: equity, value and outcomes, according to a report out Tuesday.
None of the top 20 hospitals from the U.S. News & World Report rankings made the cut to the honor roll, largely because of low grades in equity, Lown Institute said.
The report also ranked states by which had the most socially responsible hospitals. Topping the list were Hawaii, Delaware, Washington D.C., Oregon and Colorado while at the bottom were Kentucky, Kansas, Alabama, Mississippi and Arkansas.
From the human resources front
- InsuranceNewsNet informs us that “Almost one in seven Americans has absolutely no plan for their future financial and health care needs, new research suggests. A recent study of 2,000 employed Americans found that only 26% have a one- to four-year plan in place. * * * Conducted by OnePoll on behalf of Bend Financial, the study also discovered * * * more than half felt overwhelmed by the mountain of paperwork surrounding their benefit options.”
- To that end, OPM is seeking to improve the FEHB enrollment process, and FedSmith offers the first article of 2021 on the upcoming federal benefits open season. The 2022 government contribution should be released soon according to an OPM benefits administration letter.
On the business front, Beckers Payer Issues reports on senior management changes at Cigna and its Evernorth subsidiary. Among other changes, “Subsidiary Evernorth President and COO Eric Palmer was promoted to company CEO and president. Mr. Palmer will be taking over Jan. 1, 2022 for outgoing CEO Tim Wentworth, who is retiring.”
From Capitol Hill – –
- Roll Call reports on Democrat efforts to cobble together the enormous $3.5 trillion budget reconciliation bill so that a House vote on the bill can be held next week. “That ambitious timeframe, if it holds, would line up the multitrillion-dollar reconciliation bill with a vote on a smaller [one trillion] bipartisan infrastructure measure that may otherwise be defeated.”
- In better news, from the FEHBlog’s standpoint, the Wall Street Journal tells us that “House Speaker Nancy Pelosi said Congress wouldn’t let government funding expire next week, the first hint that Democratic leaders might decouple the government’s funding from a contentious increase in the debt limit, on the same day that the Biden administration began preparing for a possible partial shutdown.”