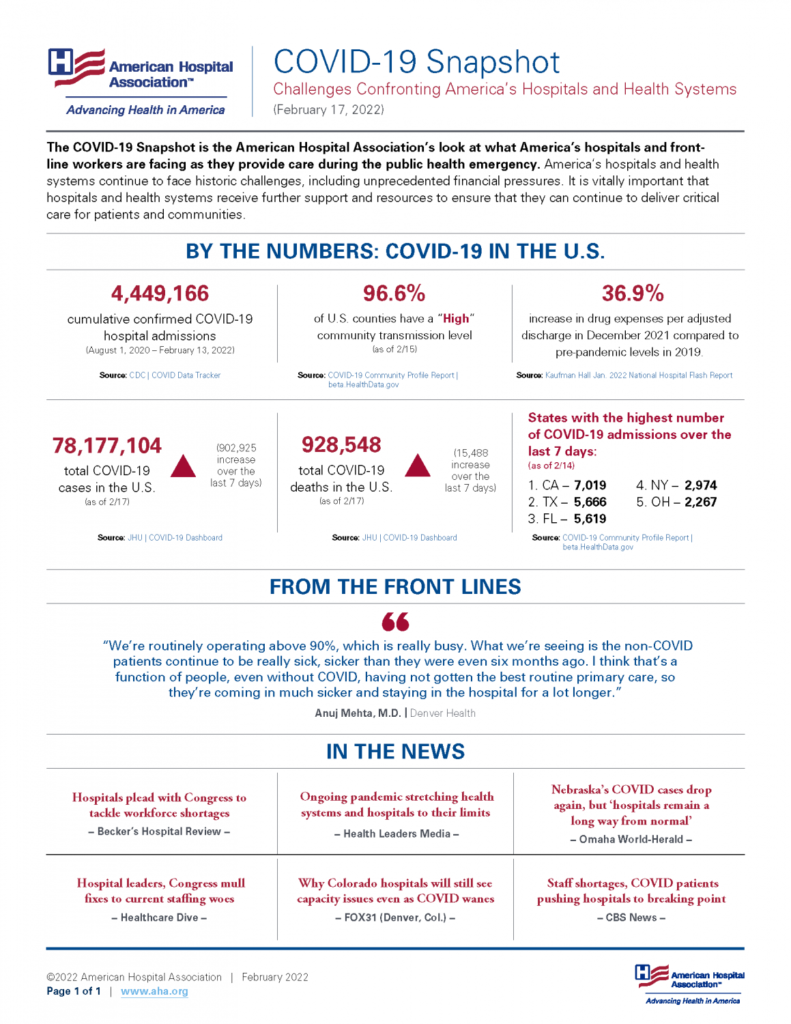
Friday Stats and More
Based on the Centers for Disease Control’s (CDC) Covid Data Tracker website and using Thursday as the first day of the week, here are the FEHBlog’s charts of weekly new Covid cases and deaths from the 27th week of 2021 through the 8th week of 2022, both of which are plummeting


Here’s the FEHBlog chart of weekly Covid vaccinations distributed and administered from the 51st week of 2020 through the 8th week of 2022iew

Here are links to the CDC’s weekly review of its Covid statistics and its weekly Fluview. The Covid news is significant
CDC is updating the way it monitors COVID-19’s impact on our communities. Widespread availability of vaccines and testing, advances in treatments, and increasing levels of immunity in the population through vaccination or previous infection have moved the COVID-19 pandemic to a new phase. While we can’t prevent all cases of COVID-19, we can continue to limit the spread and protect those who are most at risk of severe illness.
Given this new phase of the pandemic, CDC is launching a new tool to monitor COVID-19 Community Levels. Each county’s COVID-19 Community Level is ranked as low, medium, or high (find your county’s level). The COVID-19 Community Level map where you can find your county’s level will be updated regularly with new data.

Medscape adds
As of now, most counties in the country fall into either low-risk or medium-risk categories, said Greta Massetti, PhD, of the COVID-19 Response Incident Management Team.
Those counties, “representing 70 percent of Americans, are in low to medium community levels,” she said. “We continue to see indicators improve in many counties.”
A total of 23% of U.S. counties fall into the low-risk group, and 39% are medium-risk, Massetti said.
But, Walensky said, these guidelines could change at any time if numbers begin moving in the wrong direction.
Also, from the policy front
Medscape tells us and as evidenced by the new CDC approach
The White House has begun a sweeping overhaul of its COVID-19 strategy as the U.S. moves out of pandemic crisis mode and into a more manageable phase, according to ABC News.
The new strategy is expected to acknowledge that the coronavirus is becoming a less urgent threat to Americans overall due to access to vaccines, testing, and therapeutics.
Insurance News Net informs us about AHIP’s 2022 priorities as identified by its President Matt Eyles:
- Affordable coverage, improved access. Addressing the underlying cost drivers of care. Ending pharma monopolies. Addressing hospital and physician group consolidation. Pushing back on restrictions on medical management.
- Improving health equity. Ensuring everyone has an equal opportunity to thrive and achieve their best possible health. Expanding initiatives to provide health care opportunities to underserved areas and populations. Providing COVID-19 vaccine outreach to at-risk older Americans. Providing outreach in a culturally competent manner to various ethnic groups.
- Post-pandemic health care. AHIP will develop a post-pandemic road map to improve health care. AHIP is working to maintain coverage for those who were eligible for coverage under Medicaid or the Affordable Care Act during the public health emergency so that they will continue to have coverage when the emergency ends.
- Improving competition and choice. Maintain a competitive private health insurance market.
Worthy priorities, indeed.
Fierce Healthcare tells us about the American Medical Association’s President’s speech on the AMA priorities for improving U.S. healthcare and readiness for future pandemics.
From the avoiding low-value care front, Medscape reports
Low-value healthcare services that provide little or no benefit to patients are “common, potentially harmful, and costly,” and there is a critical need to reduce this kind of care, the American Heart Association (AHA) says in a newly released scientific statement.
Each year, nearly half of patients in the United States will receive at least one low-value test or procedure, with the attendant risk of avoidable complications from cascades of care and excess costs to individuals and society, the authors note.
Reducing low-value care is particularly important in cardiology, given the high prevalence and costs of cardiovascular disease in the United States, they note.
The statement was published online February 22 in Circulation: Cardiovascular Quality and Outcomes.
From the mental healthcare front, Health Affairs Forefront discusses how
This July, the US model for responding to individuals experiencing a mental health crisis is scheduled for a much-needed change. The 988 number is a three-digit, national mental health crisis hotline that was mandated by the federal government in October 2020 with an official nationwide start date on July 16, 2022. * * *
The 988 hotline holds incredible promise toward decriminalizing the response to mental health emergencies. Currently, if an individual is experiencing a mental health crisis, they, their caregivers, and bystanders have few options beyond calling 911. As a result, roughly one in 10 individuals with mental health disorders have interacted with law enforcement prior to receiving psychiatric care, and 10 percent of police calls are for mental health emergencies. * * *
Ideally, the new 988 number would activate an entirely different cascade of events. An individual in crisis, their family member, or even a bystander will be able to immediately reach a trained crisis counselor who can provide phone-based triage, support, and local resources. If needed, the counselor can activate a mobile mental health crisis team that will arrive on site to de-escalate; provide brief therapeutic interventions; either refer for close outpatient follow up or transport the individual for further psychiatric evaluation; and even offer food, drink, and hygiene supplies.
That’s an interesting perspective. Health plans should plan to publicize the 988 number this summer.
From the chronic disease front, Health Payer Intelligence uses a recent CMS report on healthcare spending to identify the ten most expenses chronic diseases in our country.
From the Rx coverage front, Healthcare Dive reports
The Federal Trade Commission is calling on the public to submit feedback on how pharmacy benefit managers’ business practices are affecting patients, pharmacies and employers.
The agency is seeking to gather a wide range of information and comments on pharmacy benefit managers, including how they affect drug prices, access, contract terms, rebates, fees, steering methods, conflicts of interest and consolidation, according to the request for information released Thursday.
Members of the public can comment through April 25. The information the FTC collects will enable the agency to “study a wide array of PBM business practices and issues and will help inform the agency’s policy and enforcement work,” the regulator said in a statement.
Finally in litigation news, the Wall Street Journal informs us
Pharmaceutical company Johnson & Johnson and three of the nation’s biggest drug distributors have agreed to move forward with a landmark settlement with a majority of states, bringing thousands of lawsuits over the opioid epidemic closer to the finish line.
Drug distributors AmerisourceBergen Corp. , Cardinal Health Inc. and McKesson Corp. would pay a total of $19.5 billion to 46 states over 18 years, according to the companies. Johnson & Johnson said it would pay $5 billion to 45 states.
The global settlement was first announced last summer. It was given final approval by the companies after a threshold number of state and local governments agreed to participate and currently amounts to roughly $25 billion.
The settlement is the largest to date from more than 3,000 lawsuits brought by states, local governments, Native American tribes, hospital groups and others alleging that companies from pharmaceutical manufacturers to distributors and pharmacies flooded areas with pills and created the opioid epidemic, ultimately forcing communities to spend millions of dollars responding to the crisis.
Let’s hope that the settlement funds are used to end our other national epidemic, substance use disorder.