Monday Musings
Today is the tenth anniversary of President Obama signing the Patient Protection and Affordable Care Act into law. The FEHBlog is tempted to muse on the law but since he has been writing in this space since 2006, he concluded no need exists for another such musing.
Be sure to check out at least the transcript for this week’s Econtalk interview with Dr. Azra Raza, a veteran oncologist who wrote a book on the human cost of cancer treatment. She explained that the benefit of early detection of cancer lies in the fact that at that point the body has fewer cancer cells that must be killed. She also touted tobacco cessation. She further explained that the new fangled CAR-T drug therapy has a weakness. CAR-T activates the body’s T cells which wind up killing healthy and cancerous tissue in a particular organ. Consequently CAR-T therapy is not used for example on liver cancer because the cure would kill the liver. Cancer is a very complicated disease.
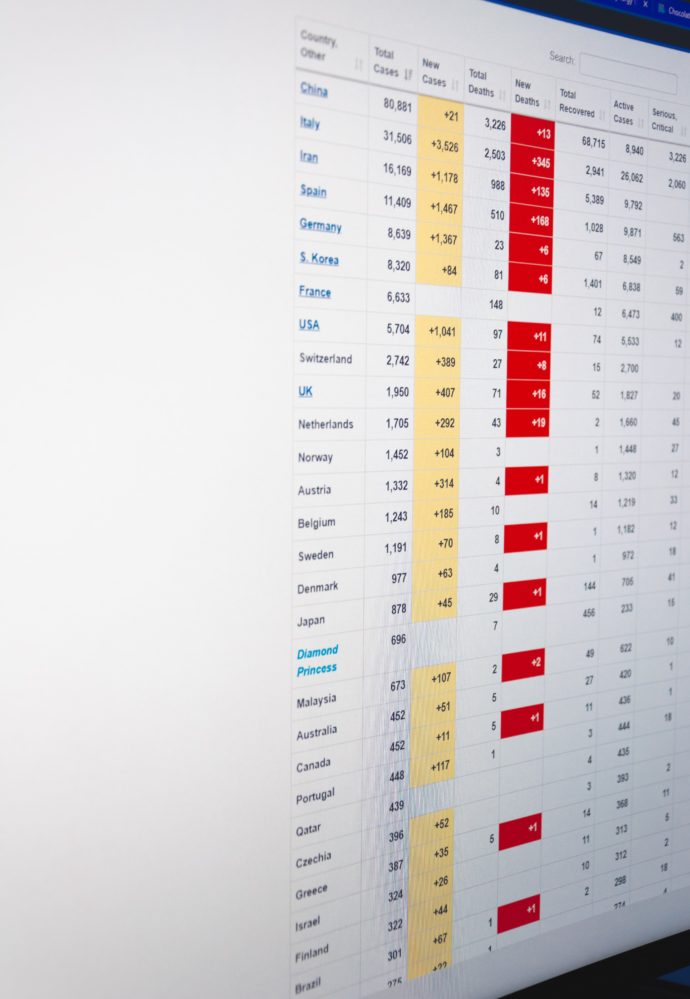
On the COVID-19 front —
- The Hill reports on continuing Senate negotiations over the third COVID-19 emergency relief bill. The American Hospital Association helpfully lists the healthcare provisions in the draft legislation which includes adjustments to the Families First relief bill’s COVID-19 testing coverage mandate and allow high deductible health plans with health savings accounts to waive their deductible for telehealth services.
- “Today the U.S. Treasury Department, Internal Revenue Service and the U.S. Department of Labor announced that small and midsize employers [under 500 employees] can begin taking advantage of two new refundable payroll tax credits, designed to immediately and fully reimburse them, dollar-for-dollar, for the cost of providing Coronavirus-related leave to their employees.”
- The Wall Street Journal offers an illuminating story about its investigation into the COVID-19 deaths at a Washington State nursing home. On February 26 the nursing home order closure of its dining rooms and an institutional scrub down due to a high number of respiratory illnesses among patients. Nevertheless the staff went ahead with a schedule party for patients, their family members and staff and ka-boom. This is why the social distancing guidance is so important right.