Midweek update

From Capitol Hill, Medtech Dive reports
The Senate Committee on Health, Education, Labor and Pensions voted Tuesday to send a bill to the Senate that would reauthorize the Food and Drug Administration to collect user fees from device- and drug-makers for the next five years.
A provision would require the FDA to finalize guidance that would create a category of over-the-counter hearing aids within a month of the bill’s passage. The FDA last issued a proposed guidance in October.
Committee Ranking Member Sen. Richard Burr, R-N.C., questioned on Tuesday whether the FDA should have that expanded authority, despite co-sponsoring legislation that would change how diagnostic tests are regulated, including laboratory-developed tests.
Fierce Healthcare adds
The American Hospital Association (AHA) penned a last-ditch letter to congressional leaders pleading for Medicare sequester cuts slated to take effect July 1 to be halted in light of the financial strain many of the nation’s hospitals are expected to face throughout 2022.
Congress had initially paused the 2% payment cut as part of the CARES Act when the COVID-19 pandemic began to threaten providers’ bottom lines. Sequestration cuts were continually punted downfield until last December, when a bill was signed to resume a 1% cut in April and the full 2% in July.
With half a month to go, AHA Executive Vice President Stacey Hughes warned majority and minority leaders Tuesday that financial relief from the pending cut is necessary for hospitals “to maintain access to care for the patients and communities they serve.”
From the Supreme Court, the American Hospital Association gleefully informs us
The U.S. Supreme Court today ruled unanimously in favor of the AHA and others, reversing a 2020 [U.S.] court of appeals decision upholding the authority of the Department of Health and Human Services to significantly cut payments to certain hospitals that participate in the 340B Drug Pricing Program, and thereby threatening access to care for patients.
The Supreme Court held that “HHS’s 2018 and 2019 reimbursement rates for 340B hospitals were contrary to the statute and unlawful.” Noting that “340B hospitals perform valuable services for low-income and rural communities but have to rely on limited federal funding for support,” the Supreme Court observed that “this case has immense economic consequences, about $1.6 billion annually.”
Despite those serious practical impacts, the Supreme Court concluded that “[u]nder the text and structure of the statute,” the case is “straightforward” as a matter of law: “Because HHS did not conduct a survey of hospitals’ acquisition costs, HHS acted unlawfully by reducing the reimbursement rates for 340B hospitals.”
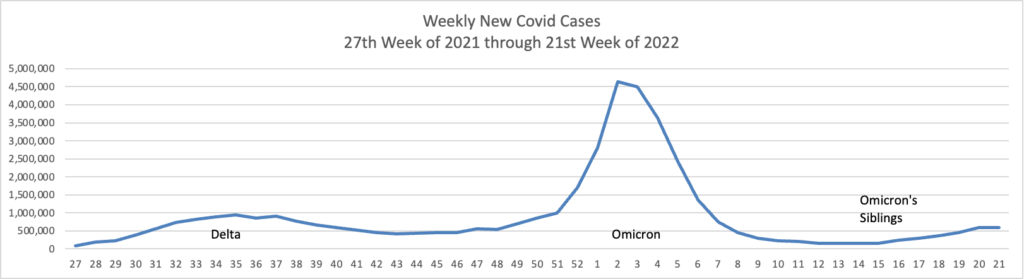
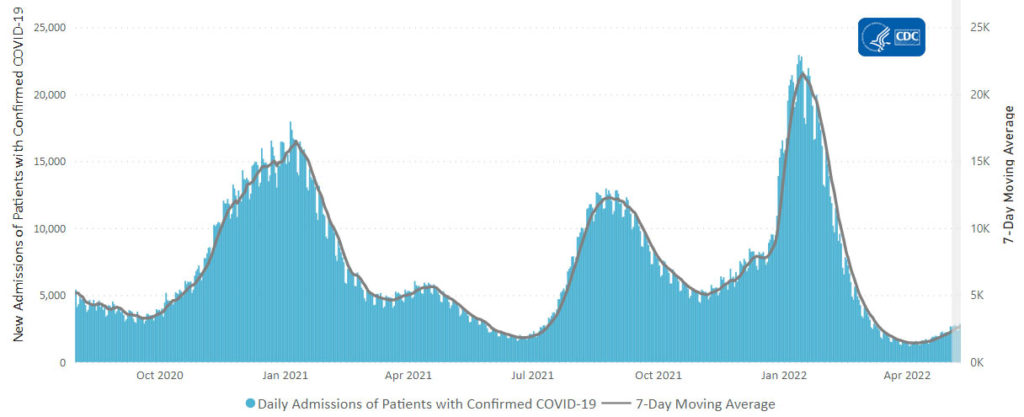
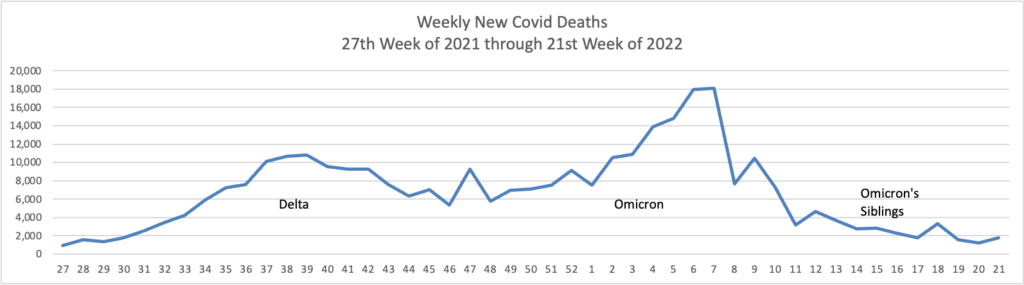
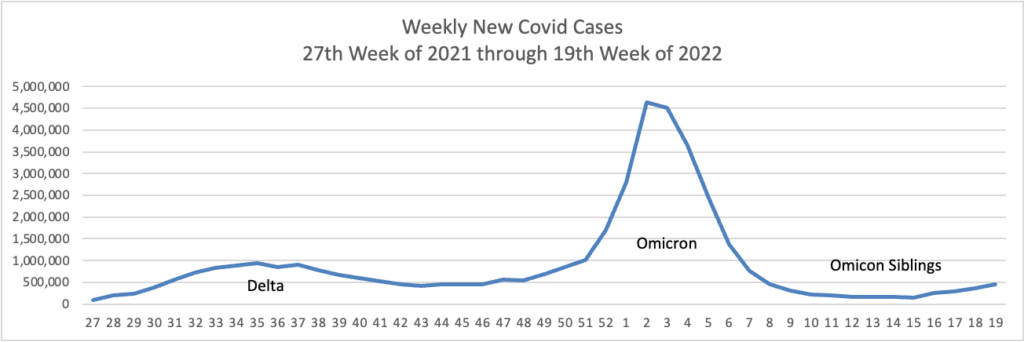
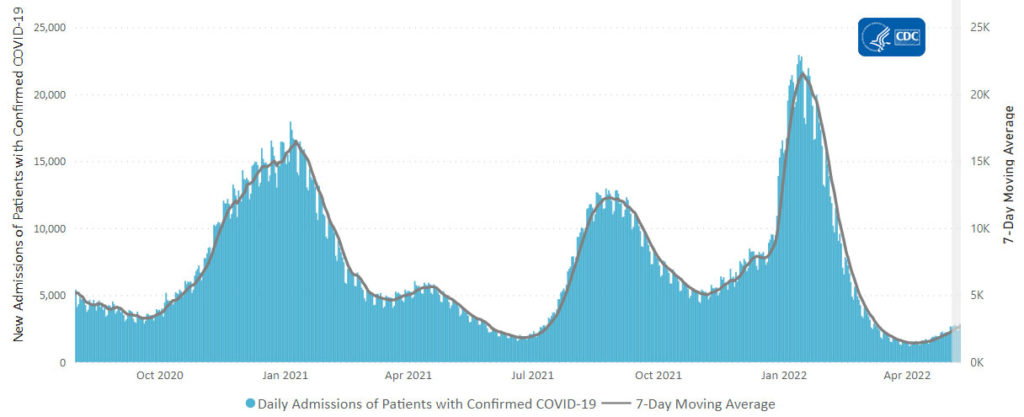
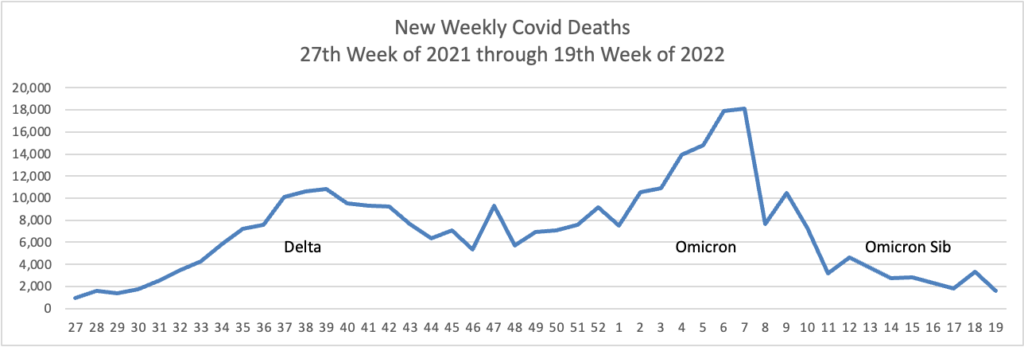
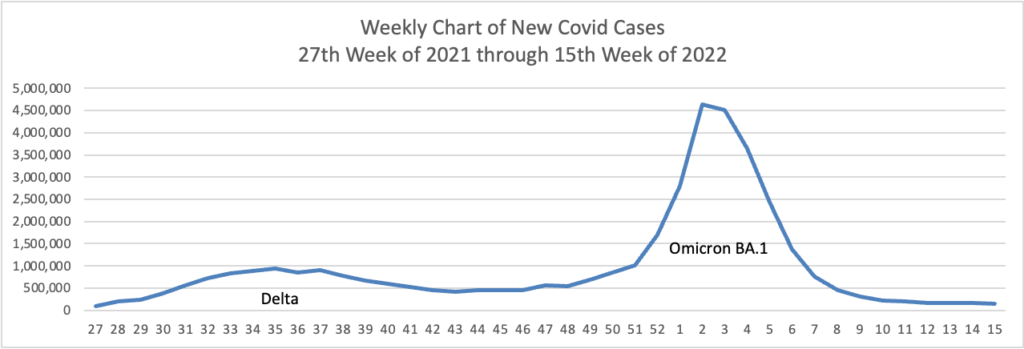
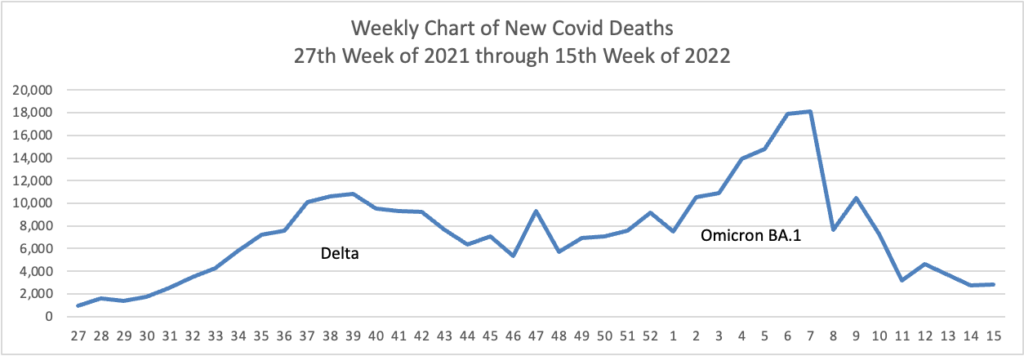
From the Omicron and siblings front —
The Wall Street Journal reports
Health experts advising U.S. health regulators backed giving Covid-19 vaccines from Pfizer Inc. and BioNTech SE and from Moderna Inc. to children as young as 6 months old.
The panel voted 21 to 0 in a pair of votes on Wednesday in support of expanding access to the vaccines.
The positive recommendations will likely lead soon to expanding the U.S. Covid-19 vaccination campaign to the 19.6 million children from 6 months to under 5 years of age, one of the last groups of people in the U.S. waiting for shots.
The Food and Drug Administration, which doesn’t have to follow the panel’s recommendations but usually does, is expected to authorize the shots within days. Vaccinations could begin as early as June 21, according to the Biden administration.
Moderna Inc. is planning to test its Covid-19 vaccine in babies 3 months to 6 months old, the youngest age group studied to date.
The Cambridge, Mass., company said Wednesday it is in the final stages of planning the study, to be called BabyCove and expected to begin enrolling as many as 700 babies in September.
BabyCove would be the first study of Moderna’s vaccine in infants younger than 6 months.
STAT News adds
Pfizer said Tuesday that a much-watched study of its antiviral Paxlovid in patients who have Covid but don’t have risk factors for severe disease failed to show a benefit in speeding alleviation of Covid symptoms, but did seem to prevent doctor’s visits and hospitalizations.
Additionally, because of the small number of hospitalizations overall in the study, it failed to produce a statistically significant finding on whether patients who had previously been vaccinated against Covid were hospitalized less often if they received Paxlovid.
The data in no way invalidate earlier results that show that Paxlovid prevents hospitalizations and saves lives in patients at high risk of severe Covid. But the results, published in a press release, are likely to take time for experts to digest and understand.
From the unusual viruses front, the American Hospital Association explains
The Centers for Disease Control and Prevention yesterday [June 14] updated its guidance to help clinicians evaluate and test patients with relevant history, signs and symptoms for monkeypox. Over 1,800 monkeypox or orthopoxvirus cases have been reported globally this year, including 72 in the United States. According to CDC, the virus does not spread easily between people without close contact, so the risk to the general population remains low.
The World Health Organization plans to change monkeypox’s name next week.
From the healthcare business front
- Per Fierce Healthcare,
Anthem will officially become Elevance Health on June 28, and, as part of its corporate rebrand, it’s also launching new brands for two of its subsidiaries.
The insurer will consolidate its healthcare services businesses under one umbrella, called Carelon. Carelon is a combination of the word “care” with the suffix “lon,” which means full or complete, representing the company’s ambition to offer an end-to-end care experience.
Carelon will include Anthem’s in-house pharmacy benefit manager Ingenio Rx as well as recent acquisitions such as Beacon Health Options, a behavioral health provider, and myNEXUS, a home healthcare company. Carelon will serve 1 in 3 people in the U.S., according to the announcement.
Humana is moving its pharmacy brands under the CenterWell umbrella.
Humana Pharmacy and Humana Specialty Pharmacy will now operate as CenterWell Pharmacy and CenterWell Specialty Pharmacy, respectively, the insurer announced. Enclara Pharmacia and Humana Pharmacy Solutions, the company’s pharmacy benefit management arm, will maintain their original branding.
“The CenterWell brand symbolizes our ongoing and strong commitment to keeping members, customers and patients at the center of everything we do,” said Scott Greenwell, Humana Pharmacy Solutions president, in a statement.
- Morning Consult discusses how CVS Health and Walgreens retained “high customer trust” in 2021.
From the benefit design front, Employee Benefits News offers the case for health savings accounts. The FEHBlog is already sold.