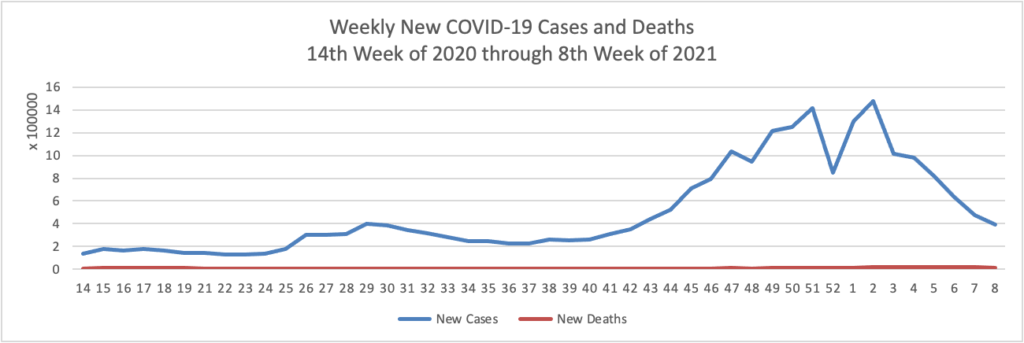
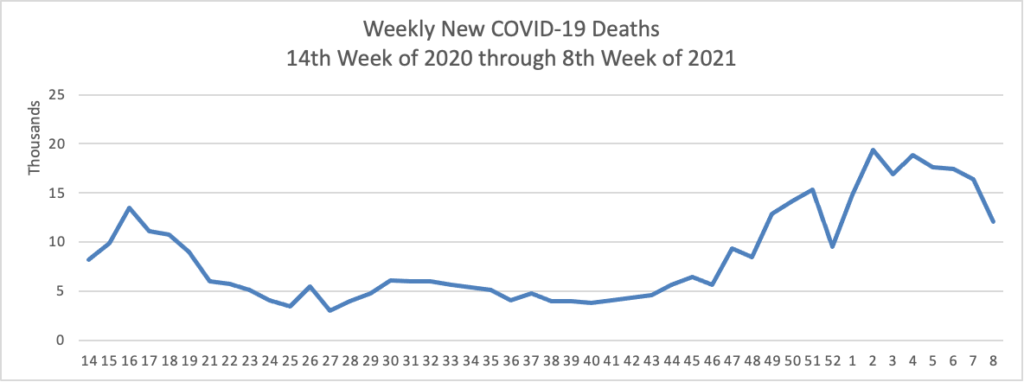
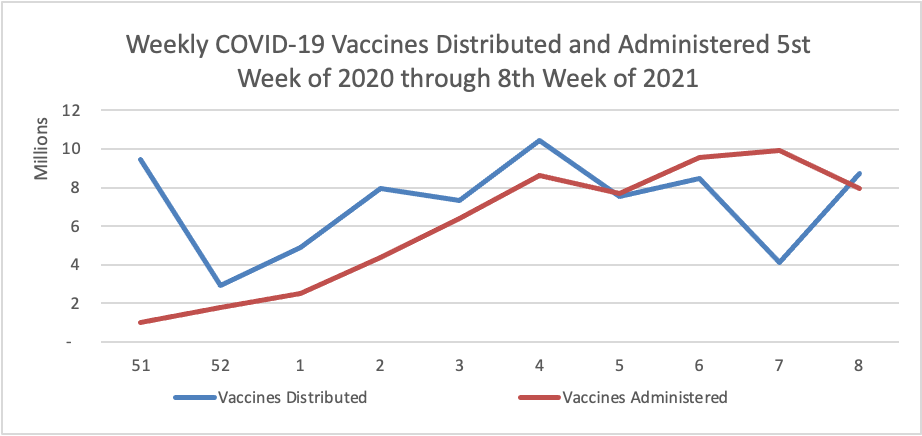
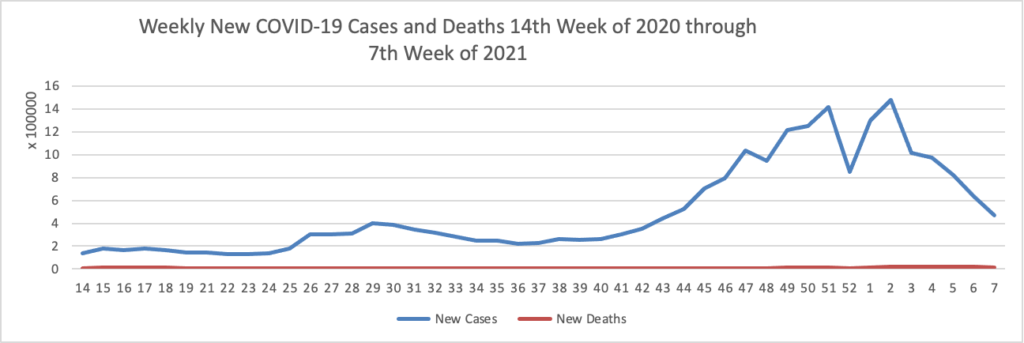
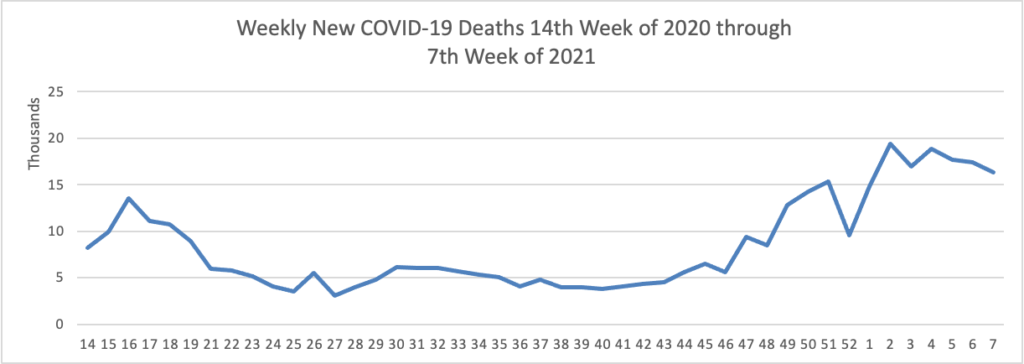
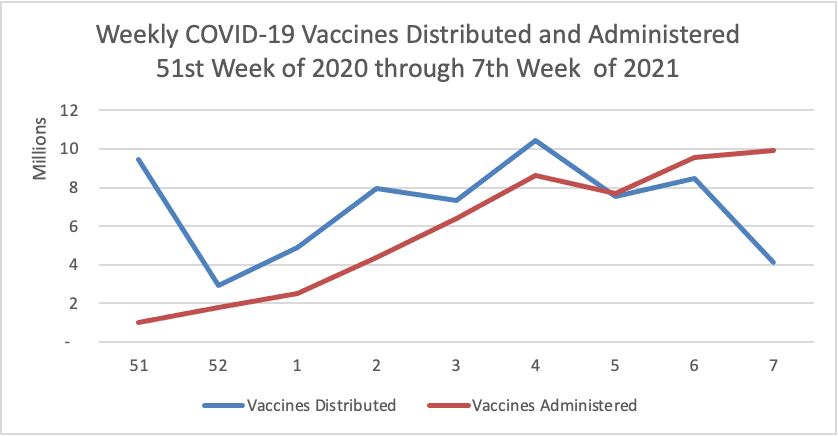
Weekend Update
Congress is session this week for committee and floor business. On Tuesday morning the House Energy and Commerce Committee will hold a hearing on “The Future of Telehealth: How COVID-19 is Changing the Delivery of Virtual Care”
The Wall Street Journal reports that
The task of passing a coronavirus relief package now rests with the Senate, where Democrats must grapple with emerging divisions over some components of the plan, including a minimum-wage increase. The House early Saturday morning passed [largely along party lines] President Biden’s $1.9 trillion package, which would fund vaccine distribution, enhance and extend federal unemployment benefits, and send direct checks of $1,400 to many Americans and $350 billion to state and local governments.
As mentioned in last Thursday’s post, the Democrat leadership in Congress is trying to figure out a way for the Senate to pass the entire bill under budget reconciliation which requires all fifty Democrat senators plus the Vice President. The $15 minimum wage provision found in the House bill remains a wild card in the Senate.
The President officially sent Kiran Ahuja’s nomination to be OPM Director to the Senate last Wednesday. Federal News Network forecasts six “challenges” that Ms. Ahuja will need to address once confirmed.
In most excellent news, the Food and Drug Administration gave emergency use authorization to the single dose Johnson and Johnson vaccine yesterday and the Centers for Disease Control seconded this action today. This means that health plans, including FEHB plans, become liable for reimbursing administration costs for the Johnson and Johnson vaccine without member cost sharing in 15 days / March 15, 2021. Per CNN with the blessing of these two agencies
[T]he federal government may then begin distributing the 3.9 million available doses of the vaccine, perhaps as soon as Monday.”I just want to state explicitly how very grateful I am that we now have three highly effective vaccines,” said ACIP member Dr. Matthew Daley of the Institute for Health Research with Kaiser Permanente Colorado.
The company has pledged to have 20 million doses available by the end of March and 100 million doses by summer.The vaccine, made by Johnson & Johnson’s Janssen vaccine arm, can be kept at regular refrigerator temperatures, which experts said would make it much easier to distribute than vaccines made by Moderna and Pfizer/BioNTech.
The Wall Street Journal sums it up for us as follows:
The pandemic has opened a new era for vaccines developed with gene-based technologies, techniques that have long stumped scientists and pharmaceutical companies, suggesting the possibility of future protection against a range of infectious disease.
Johnson & Johnson’s Covid-19 vaccine, which was authorized Saturday for use in the U.S., is at the vanguard of a class of shots designed to mobilize a person’s immune defenses against the disease. It will be the first Covid-19 vaccine administered in the U.S. that uses viral-vector technology, which employs an engineered cold virus to ferry coronavirus-fighting genetic code to the body’s cells.
J&J’s vaccine is the third to be authorized in the U.S. after ones from Pfizer Inc. and its partner, BioNTech SE, and Moderna Inc. In a late-stage trial, J&J’s single-shot vaccine was 66% effective in preventing moderate to severe cases of the disease that has killed more than 500,000 people in the U.S. and about 2.5 million world-wide.
“This is one of those giant leap moments for us. These are fundamental shifts in how we will build vaccines for the future,” said C. Buddy Creech, director of Vanderbilt University’s vaccine research program. “I think this really ushers in a golden age of vaccinology.”
By the way the Centers for Disease Control has created its own COVID-19 vaccine finder website. According to the CDC’s COVID-19 data tracker website, currently nearly 20% of the eligible U.S. population has received at least one dose of the vaccine and 10% have received both doses.
Finally, the Choosing Wisely campaign is offering a information and a webinar that address one of the points in OPM’s recent call letter for 2022 benefit and rate proposals from carriers:
In Building A Better Health Care System Post-Covid-19: Steps for Reducing Low-Value and Wasteful Care, Corinna Sorenson, PhD, Duke-Margolis Center for Health Policy, and colleagues outline the impact of the pandemic on low-value care, and the potential opportunities it presents to create a better health care system post COVID-19. She elaborates further on this topic in her January 2021 Choosing Wisely webinar recording.