Weekend update

Happy Easter!
Congress remains on State/district work breaks for the coming week.
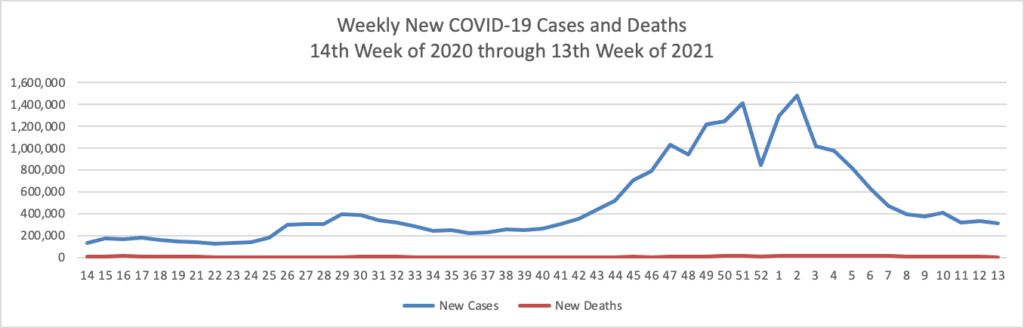
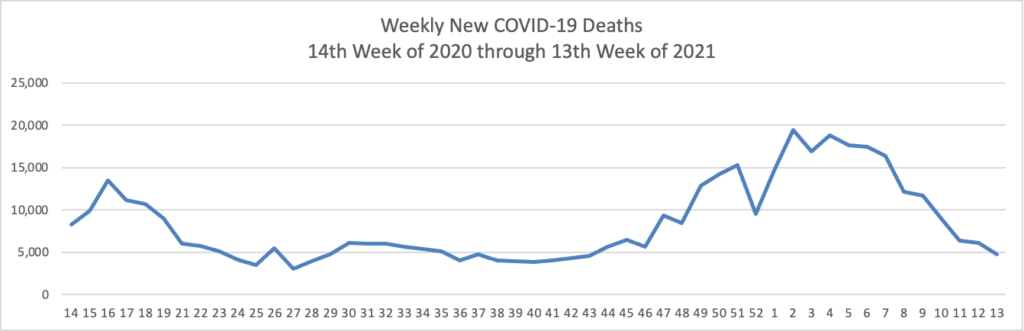
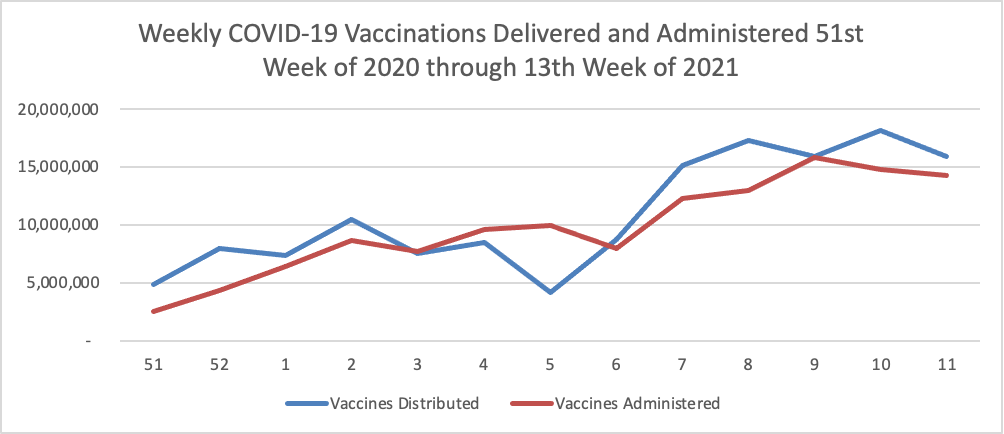
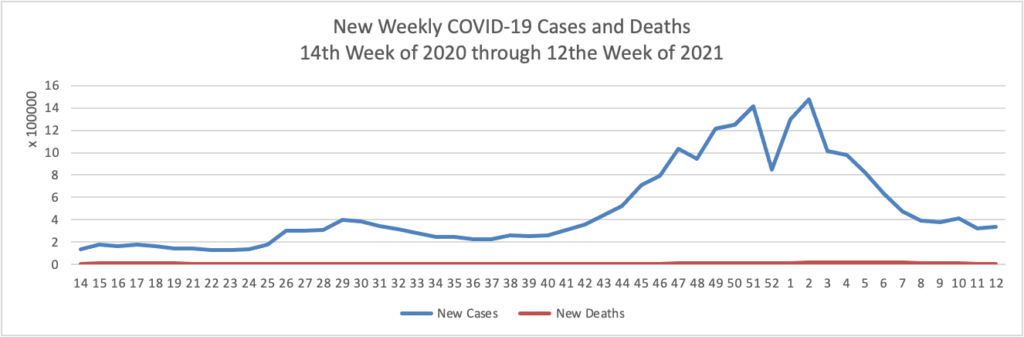
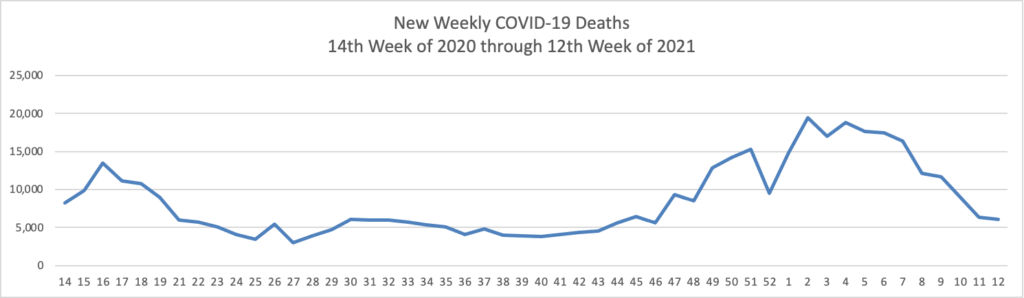
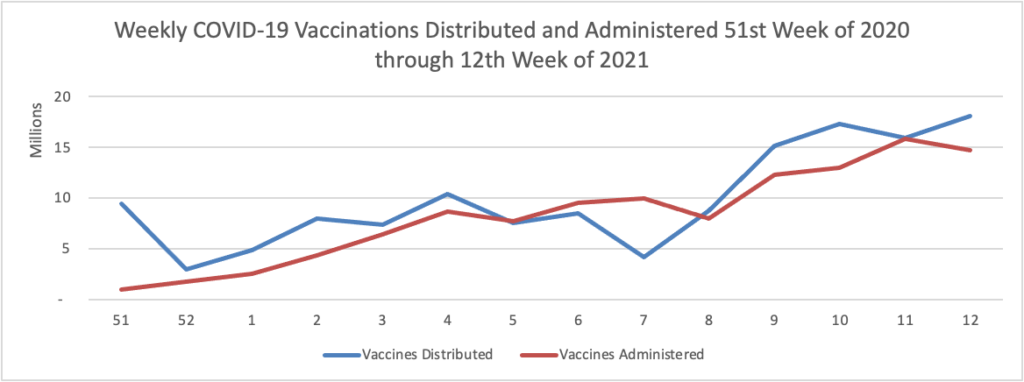
As of the beginning of this week, according the CDC’s website, 75.4% of the U.S population over age 65 and 40.2% of the U.S. population over age 18 has received at least one dose of the COVID-19 vaccine. 54.5% of the over age 65 population and 23.2% of the over 18 age population have been fully vaccinated.
That’s progress. Here are a couple of interesting angles on the vaccine distribution process:
- The Wall Street Journal reports that “Johnson & Johnson’s Covid-19 vaccine has found a niche among organizations that work with the homeless, who say the one-dose shot is better-suited for a population that can be difficult to reach twice.”
[H]ealthcare workers say they have been surprised to find many homeless people specifically requesting the J&J vaccine, which is branded as Janssen, a unit of J&J. Some of them point out that the shot was still effective even though it was tested after Covid-19 variants entered the mix. Others say they are worried about getting a vaccine once, let alone twice, given the potential side effects.
“If you’re in a shelter, or don’t have a home, those side effects are different than if you can stay at home,” said Bobby Watts, chief executive of the National Health Care for the Homeless Council, which supports hundreds of providers that cater to the homeless.
- Health Payer Intelligence informs us that “To ensure COVID-19 vaccine access for homebound individuals, the Commonwealth of Massachusetts has partnered with the Commonwealth Care Alliance (CCA), a health plan that says it has proven best practices for vaccinating this population.” “The Commonwealth defines a “homebound” individual as anyone who needs assistance from two or more people to leave home. In Massachusetts, there are about 20,000 individuals who meet this definition.”
As one of the first healthcare organizations in the country to vaccinate homebound individuals, CCA has also been part of the national discussion around strategies to ensure COVID-19 vaccine access for this population. Last month, CCA joined AHIP in briefing the White House, promoting the prioritization of homebound individuals in COVID-19 vaccine delivery efforts and underlining CCA’s best practices in this endeavor.
Speaking of AHIP, the organization on Friday announced
AHIP’s new SEP landing page also features other important resources to help guide consumers through the SEP, including fast facts, an educational blog, a link to a Get Covered Connector tool offered by Young Invincibles, and a link to a Health Insurance Marketplace Calculator provided by the Kaiser Family Foundation.
“Health insurance coverage is an important way to protect your health and financial stability, especially during the COVID-19 pandemic,” said Matt Eyles, president and CEO of AHIP. “Health insurance available through the individual marketplaces cover products and services such as COVID-19 care and vaccines, mental health care and support, $0 copay preventive care, regular doctor visits and prescription medications to keep you healthy, and much more.”
In other COVID-19 news, Kaiser Health News provides details on over-the-counter COVID-19 testing kits.
Even with vaccines, epidemiologists say, rapid tests are desperately needed because more testing, along with mask-wearing and physical distancing, will get people back in offices and classrooms and help catch cases that go undetected. * * *
[M]any experts support the widespread distribution of cheap, rapid tests, even if they aren’t as sensitive as lab-run alternatives, and see a demand. In Germany, the supermarket chain Aldi began selling rapid tests in early March, roughly $30 for a five-pack, and sold out within hours. One recent study found that if a pack of tests was mailed to every household in the U.S. — even assuming that up to 75% would go into the garbage — they would save thousands of lives and avert millions of infections. “Don’t let perfect be the enemy of good,” said study co-author and Yale University professor A. David Paltiel. “This doesn’t have to work perfectly to make a huge difference.”
The Federal News Network shares opinions that it obtained from former OPM officials on the recent National Academy of Public Administration report on the agency.
Janice Lachance, the Clinton-era OPM director, sees the budget as a good starting point for the Biden administration and the new director. The president nominated Kiran Ahuja, a former chief of staff for the agency, for the role.
“The new director has a tremendous opportunity to go in there, do a very effective assessment of the situation and make a reasonable request that covers all of the things that need to be done — and that we want to do,” said Lachance, who currently serves as an executive vice president for the American Geophysical Union. “The NAPA report is very aspirational. What is it going to take to get OPM from where it is today to this desired state that’s articulated in the NAPA report over how many years?”
The new director, Lachance added, will need to make the case why an empowered OPM will help resolve the federal government’s talent problems.