Friday Stats and More
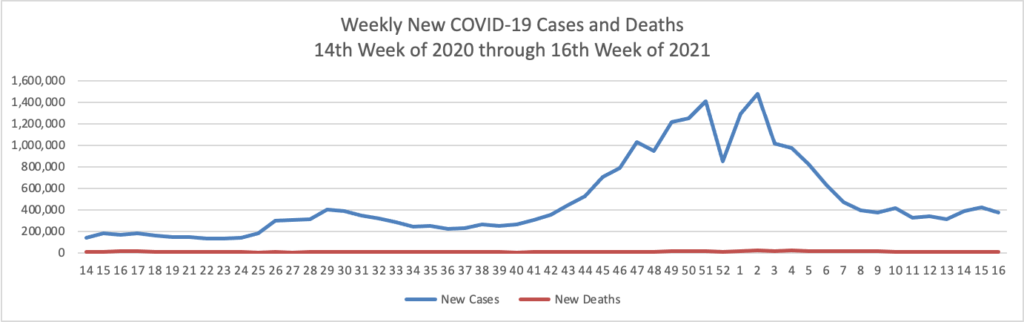
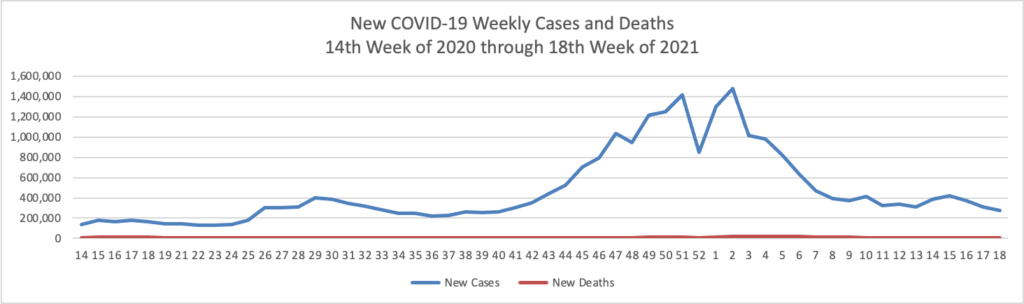
Based on the Centers for Disease Control’s COVID-19 Data Tracker website, here is the FEHBlog’s chart of new weekly COVID-19 cases and deaths over the 14th week of 2020 through 18th week of this year (beginning April 2, 2020, and ending May 5, 2021; using Thursday as the first day of the week in order to facilitate this weekly update):
and here is the CDC’s latest overall weekly hospitalization rate chart for COVID-19:

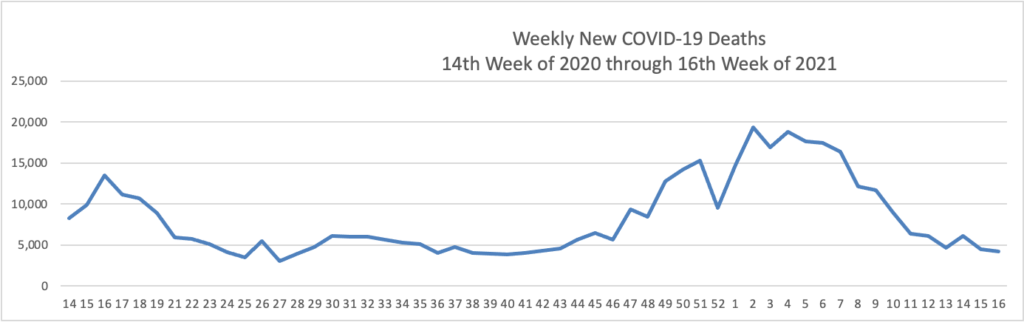
The FEHBlog has noticed that the new cases and deaths chart shows a flat line for new weekly deaths because new cases greatly exceed new deaths. Accordingly here is a chart of new COVID-19 deaths over the period (April 2, 2020, through May 5, 2021):
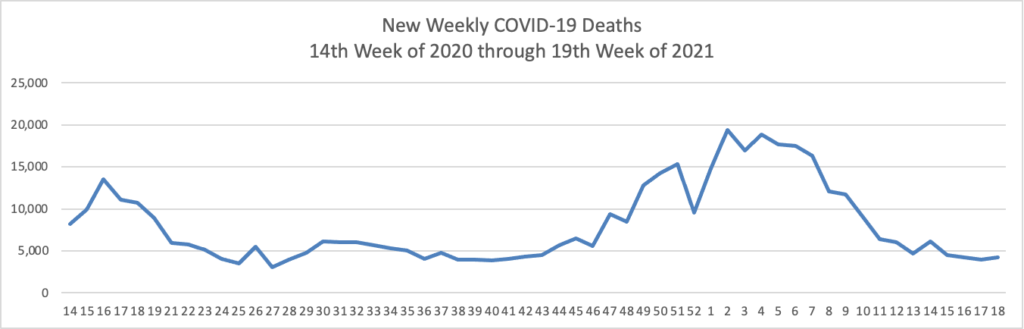
The Wall Street Journal observes and the charts evidence that
The U.S. may finally have turned a corner in the Covid-19 pandemic, according to epidemiologists and public-health officials.
Newly reported coronavirus cases have declined for three straight weeks, and the seven-day average of Covid-19 PCR tests that returned positive is hovering around 4%, one of its lowest points in the pandemic. Hospitalizations have been declining and reported deaths have fallen every week since late March.
The decrease in nearly every key metric comes as the U.S. meets a benchmark in its vaccination campaign. More than 40% of the adult population is now fully vaccinated, which many public-health experts say is an important thresholdwhere vaccinations gain the upper hand over the virus.
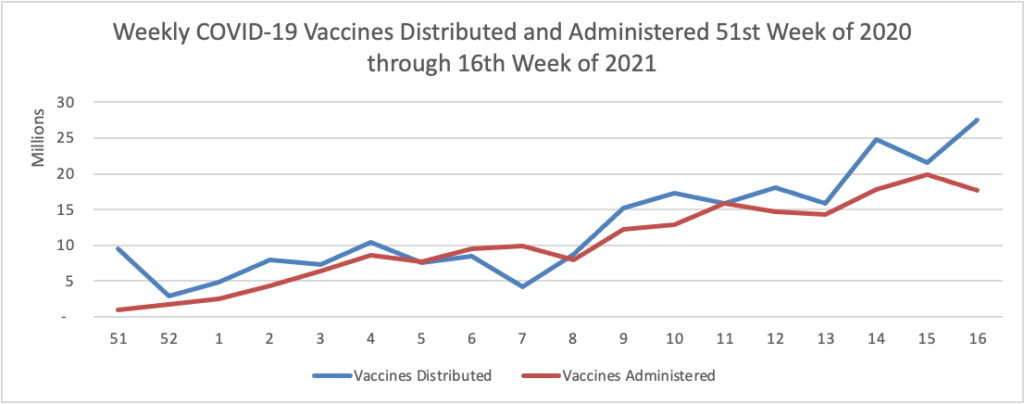
Here is a weekly COVID-19 vaccinations chart over the period December 17, 2020, through May 5, 2021, which also uses Thursday as the first day of the week:

The Centers for Disease Control observes
Following a rapid acceleration in vaccination rates, we are now seeing U.S. vaccination progress slow. This is not surprising considering the prior focus on vaccinating people at increased risk. Also, people eager to be immunized when they became eligible may have already secured their vaccine in line with increased supply. While more than 8 in 10 people 65 years and older have received at least one dose of vaccine, only around 1 in 3 people ages 18-29 have. All age groups currently eligible for the vaccine can benefit from the protection it provides themselves and others, especially as more states are easing prevention measures.
Also from the COVID-19 vaccination front:
- The Society for Human Resources Management provides helpful guidance to employers on how to help achieve herd immunity.
- The CDC’s Advisory Committee on Immunization Practices will vote on Wednesday May 12 on whether to extend Pfizer’s emergency use application for its COVID-19 vaccine to children ages 12-15.
- The Wall Street Journal reports that “AstraZeneca PLC could skip asking the Food and Drug Administration for emergency-use authorization for its Covid-19 vaccine, according to people familiar with the matter—and instead pursue the more time-intensive application for a full-fledged license to sell the shot.”
- Law professor Richard Epstein weighs in on the hot topic of “Intellectual Property and the COVID-19 vaccines.”
From the healthcare business front
- Healthcare Dive reports on Cigna’s 1st quarter 2021 results. The health insurer “beat Wall Street expectations in the quarter, and increased its forecast for the full year, signaling optimism for the remainder of 2021 despite the ongoing uncertainty.”
- Fierce Healthcare reviews several health insurers’ first quarter 2021 results.
In other news —
- The FEHBlog understands why according to Becker’s Payer Issues, 95% of insurers “are worried about meeting No Surprises Act requirements by [the January 1, 2022] deadline. Congress created an overcomplicated law. Hopefully the regulators can straighten it out in time.
- The American Hospital Association questions the Lown Institute report on low value hospital care that the FEHBlog mentioned earlier this week.
- Health Payer Intelligence brings us up to date on electronic attachments to HIPAA standard claims transactions, the one HIPAA requirement that HHS has not been able to tackle successfully.
- Strangely, a British website helpfully summarizes the path of Kiran Ahuja to become OPM Director. “At her hearing, Ahuja said: “I believe people are, and should be, at the centre of all policy decisions, and… I would carry forward this guiding principle while working in service to the American public.” It remains to be seen whether the Senate, in a time of division, accepts that Ahuja can be the unifier the US public service needs.” My bet remains on confirmation.