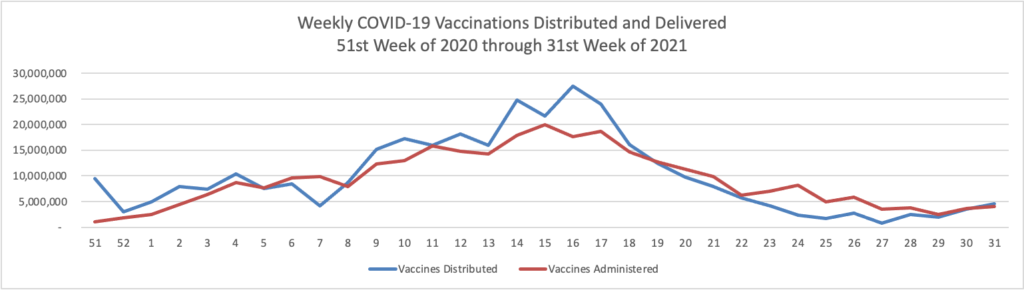
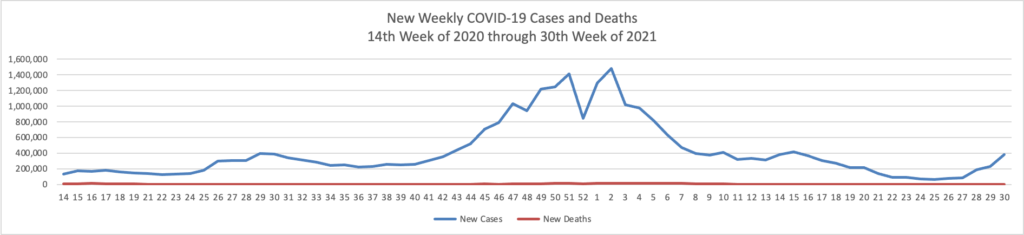
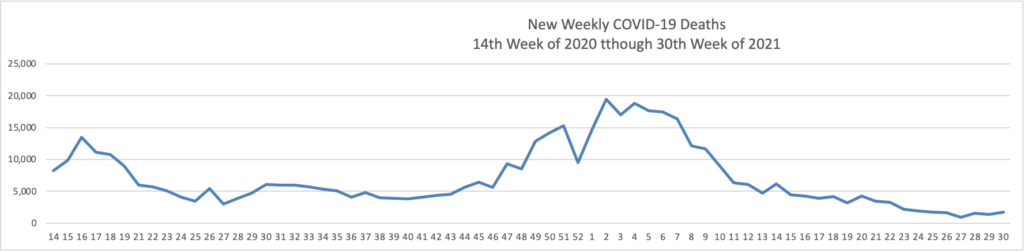
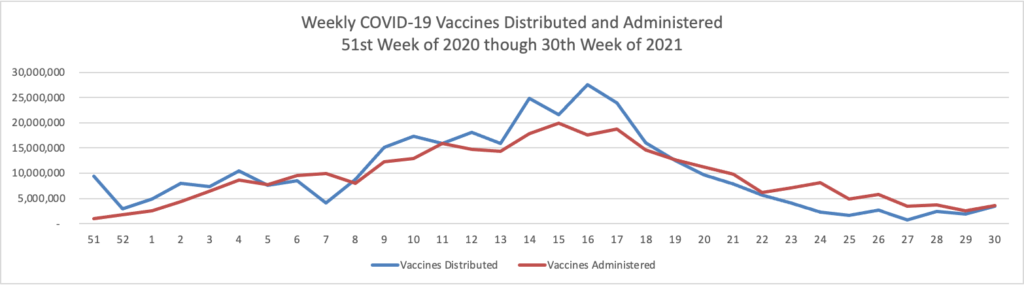
Monday Roundup

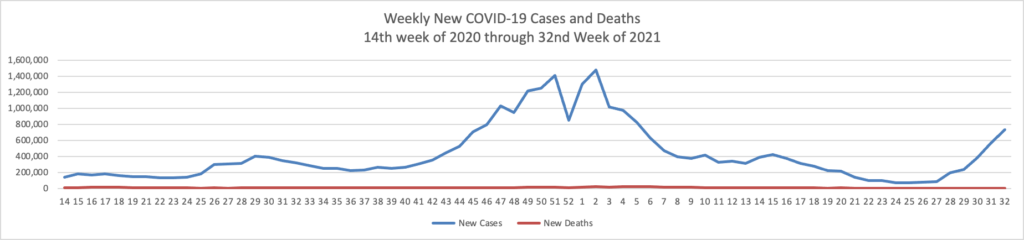
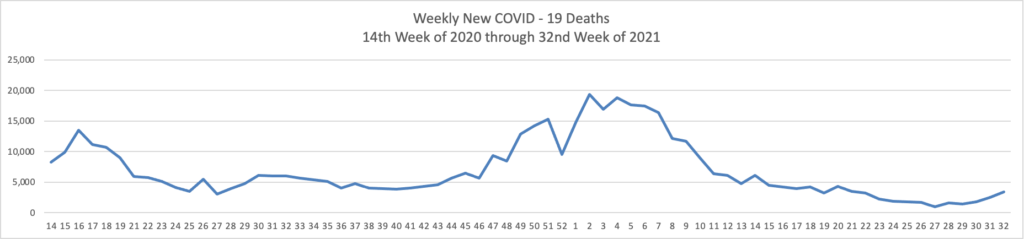
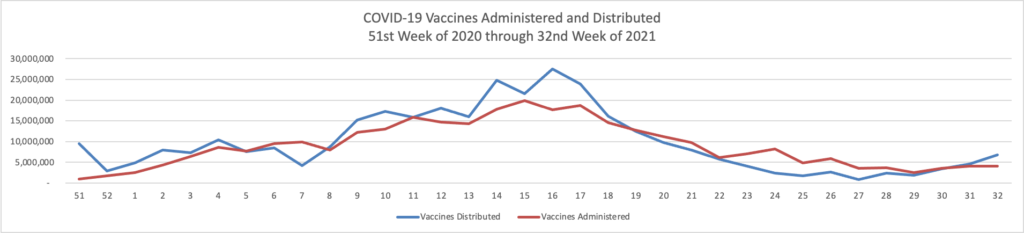
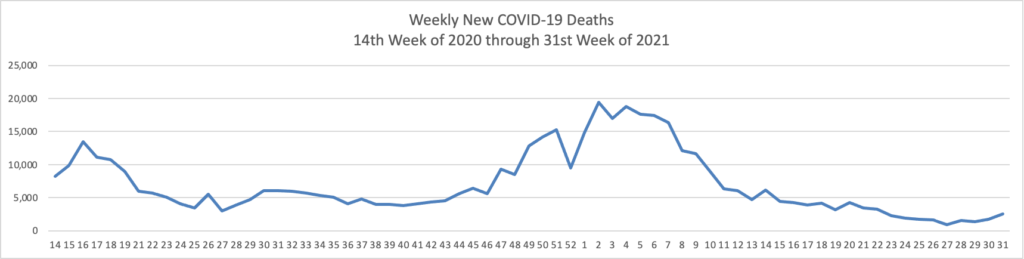
From the Delta variant front —
- Govexec tells us that last Friday August 13, the Department of Justice issued its internal guidance on employee, on-site contractor, and visitor COVID-19 vaccine attestation program required by the White House. ‘Federal employees, regardless if they are working in person or teleworking, must complete a Certification of Vaccination Form and update it if there is a change in their vaccination status. ‘Employees who are not fully vaccinated will be required to obtain and provide to their supervisor (or component designee) a negative COVID-19 test result, from a test taken within the past three days, each time they enter a department facility or participate in an official meeting or function in another location other than the telework location,’ said the memo. ‘The department is developing a program to facilitate testing for employees.’”
- The American Medical Association informs us about the reasons why moderately and severely immuno-compromised Americans who compose 2.7% of the population should get an mRNA vaccine booster. “[Food and Drug Administration] Approval of a third dose comes amid growing evidence that people with weakened immune systems do not get adequate protection from the normal two-dose regimen of Pfizer and Moderna COVID-19 vaccines. This makes immunocompromised people especially susceptible to breakthrough COVID-19 infections.”
- The Wall Street Journal reports that Pfizer and BioNTech are starting to share clinical evidence of the value of a third booster vaccination for a broader swath of the U.S. population administered six months to a year following the first two doses. “Pfizer and BioNTech are also conducting a larger late-stage study evaluating whether a third dose safely provides more protection. The companies said they expect those results shortly and will then submit the data to the FDA. The FDA is considering a broader booster strategy, which the agency could issue in the next few weeks.”
With regard to the other public health emergency, the American Hospital Association points us to it web resources for the addressing opioid epidemic.
In healthcare business news
- Labcorp has announced the acquisition of “Ovia Health, a digital health platform used by millions of women seeking information and support with family planning, pregnancy and parenting.”
- CareMax, a “technology-enabled provider of value-based care to seniors, announced [on August 13] it has signed a collaboration agreement with Anthem, a national health benefits company. Through this collaboration, CareMax plans to build medical centers in areas where Anthem will offer a value-based care model to improve patient outcomes. * * * Through this collaboration agreement, CareMax plans to open approximately 50 medical centers with a focus on Indiana, Texas, Kentucky, Wisconsin, Georgia, Connecticut, and Virginia, among others.”
From the studies front
- The University of Michigan’s Institute for Healthcare Policy and Innovation has released an eye opening study on telehealth. The FEHBlog commends it to his readership.
- Benefits Pro tells us that “Interest in health savings accounts is on the rise, but there is still a gap in understanding about how HSAs work and how they can be used as a retirement savings tool. According to the Plan Sponsor Council of America’s (PSCA) 2021 HSA Survey, education about HSAs remains a key goal for plan sponsors. * * * The study pointed to a potential missed opportunity for employers to solicit HSA rollovers for newly hired employees, with less than 20 percent indicating they do so.”
- The National Institutes of Health informs us that “A single two-hour session of a pain management skills class could offer as much benefit as eight sessions of cognitive behavioral therapy (CBT) for patients experiencing chronic low-back pain (CLBP), suggests a study published in JAMA Network Open(link is external). Supported by the National Center for Complementary and Integrative Health (NCCIH) and the National Institute on Drug Abuse, both part of the National Institutes of Health, the study explored whether a compressed intervention could lead to the same benefits as a longer-course of CBT.”
- STAT News reports that the National Institute for Mental Health’s “Director Joshua Gordon told STAT the agency recognizes that new treatments for depression are needed and that it is funding research to explore the underlying biology beyond the monoamine hypothesis. When it comes to understanding depression, “there are fewer and fewer questions of importance with regard to the monoamine systems,” he said. He noted that in addition to federal funding, many startups and small companies are pursuing new treatments for depression. Currently, almost all patients with depression are first treated with medications called selective serotonin reuptake inhibitors, a class of drugs that includes both Zoloft and Prozac. They increase the amount of the neurotransmitter serotonin in the brain, which controls mood, emotions, and cognition. Serotonin and two other neurotransmitters targeted by some antidepressants, norepinephrine and dopamine, are called monoamines because they contain one chemical group called an amine.” The article also discusses those new treatments, including .