Tuesday’s Tidbits

Roll Call discusses the status of the $3.5 trillion budget reconciliation bill as “Senate Democrats begin to tweak the House package.” Federal News Network provides a broader perspective on the varied issues facing Congress this month.
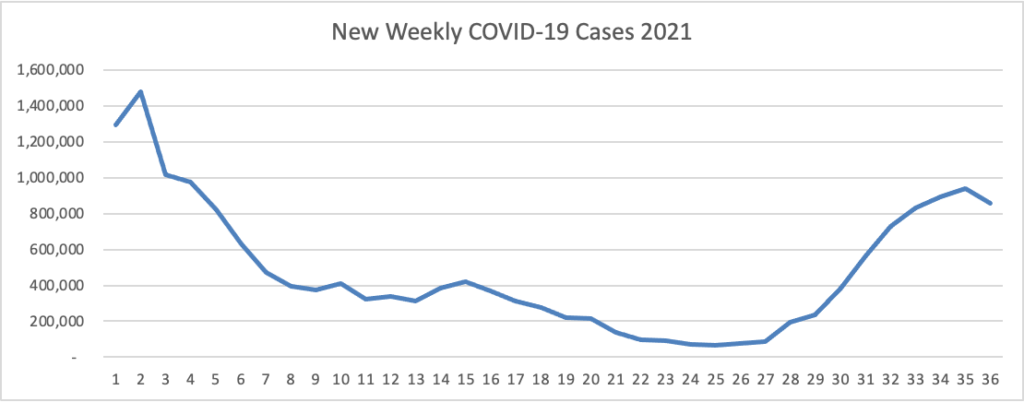
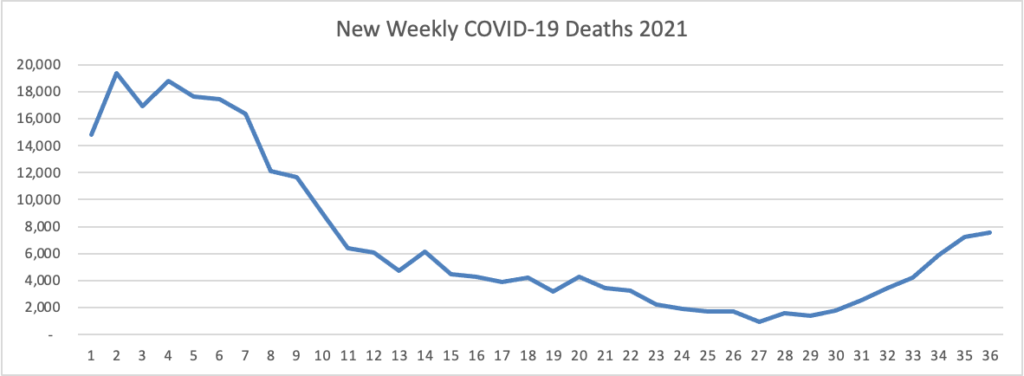
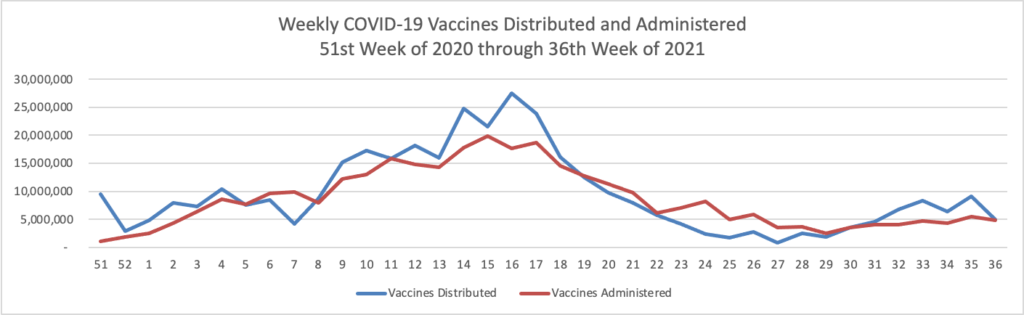
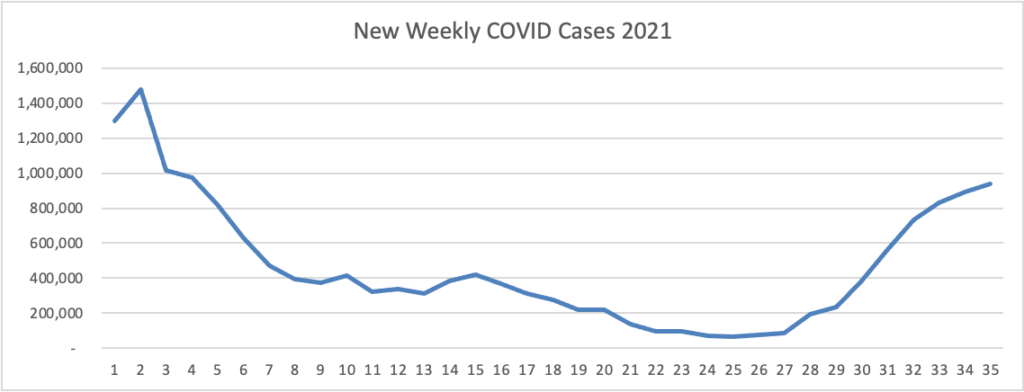
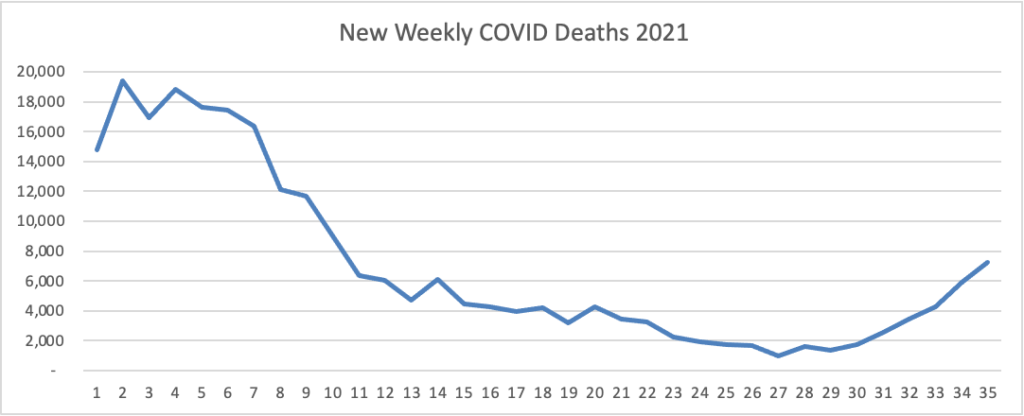
From the Delta variant front
- The Department of Health and Human Services” (HHS) Vaccination Community Corps has updated its resources list.
- Yesterday, HHS “transitioned from a direct ordering process to a state/territory-coordinated distribution system * * * for the distribution of monoclonal antibody drugs (“mAbs”) [to treat COVID-19 particularly in unvaccinated people]. State and territorial health departments know best where product is needed in their areas. Transitioning to a state/territory-coordinated distribution system gives health departments maximum flexibility to get these critical drugs where they are needed most.” Makes sense.
- Healthcare Dive reports that “Just as patients started returning for care they delayed throughout the COVID-19 pandemic, hospitals are again having to put non-emergency procedures on hold to free up resources for patients hospitalized with the delta variant of the coronavirus. Salt Lake City-based Intermountain Healthcare is the latest system to announce it’s postponing elective procedures starting Wednesday at 13 of its hospitals, and expects the pause to last several weeks, CEO Marc Harrison said during a Friday press conference.”
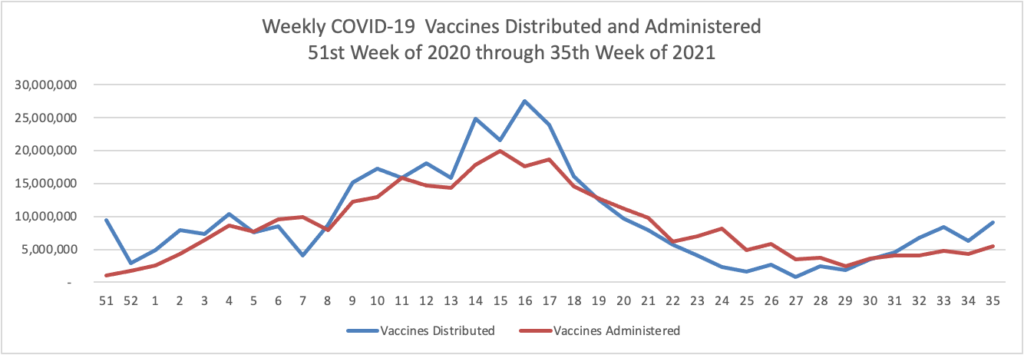
- JDSupra offers a useful and in the FEHBlog’s opinion accurate legal analysis of the President’s vaccination programs for federal contractors and private employers. Bloomberg expresses concern that “The U.S. may not have enough tests to keep pace with the Biden administration’s tightened workplace Covid-19 mitigation measures.”
In the tidbits department
- The Mercer consulting firm announced that ‘[a]s the pandemic continues to unfold, the ability of employers to have a positive impact on employee health and resiliency cannot be understated and is one of the most important findings of the latest Mercer “Health on Demand” survey released today.” Since the onset of COVID-19, when employers stepped up to provide essential support, it made a difference. Employees who say they received good support from their employers are much less likely to view their personal experience of the pandemic as mostly or entirely negative compared to those who received little or no support – 25% vs. 49%. And almost half (45%) of those receiving good support say they are less likely to leave their job as a result.
- The FEHBlog noticed a Modern Healthcare article discussing how employers are incorporating virtual substance abuse programs in their provider networks. As an example the article pointed to Ria Health which describes itself as an evidence based alcohol additional treatment program.
- The American Medical Association’s President (appropriately in the FEHBlog’s view) is urging physicians and patients to reengage. “As life takes steps to return to normal, it’s urgent that patients and physicians prioritize health screenings and regular procedures.”