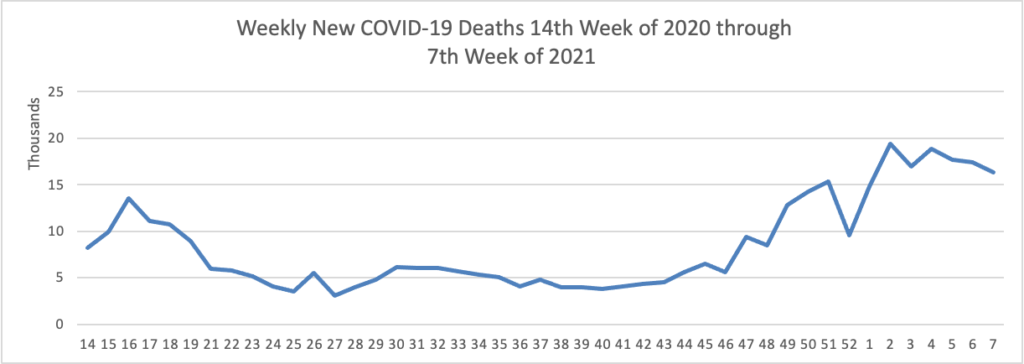
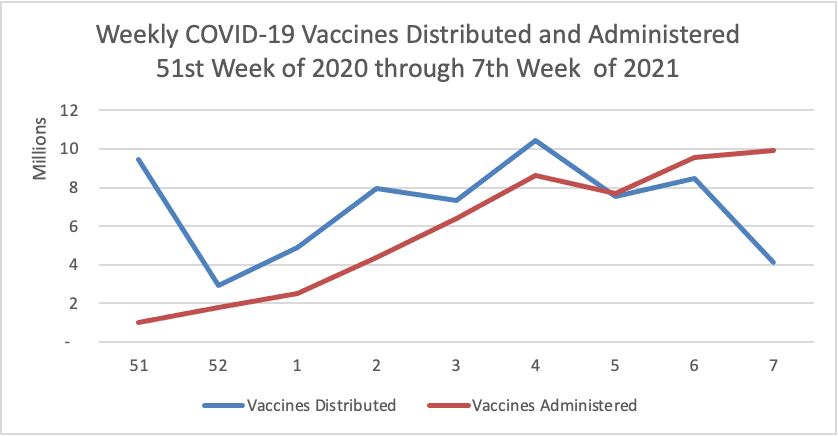
Midweek update
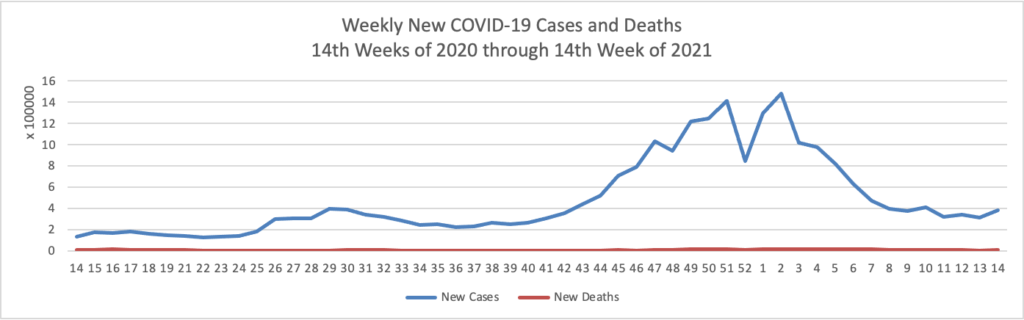
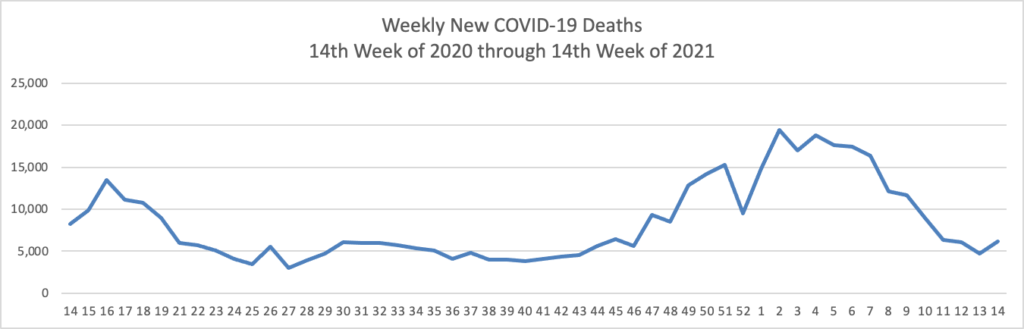
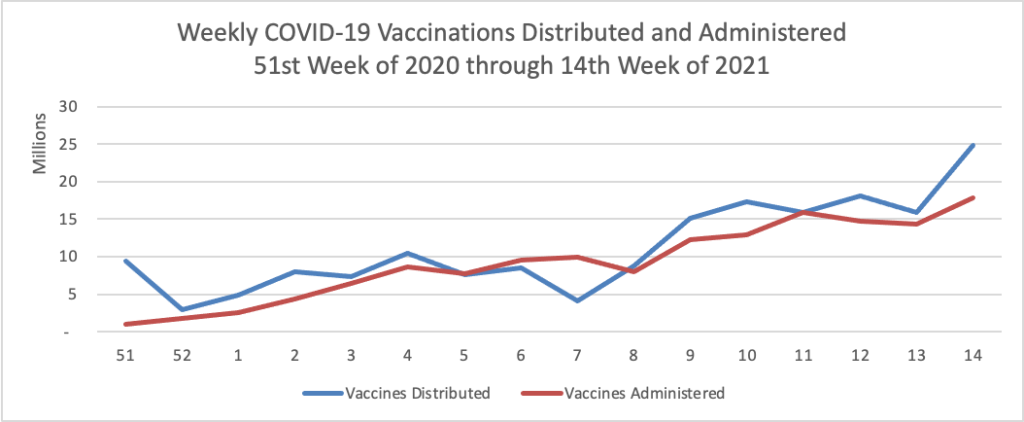
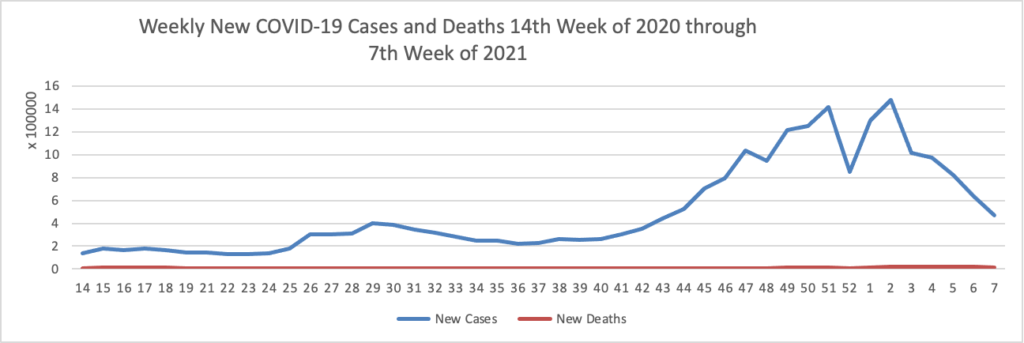
Today was the second day of the OPM AHIP FEHB Carrier Conference. One of the sessions concerned COVID-19 vaccination outreach to socially disadvantaged communities. The FEHBlog learned that Kaiser Permanente, which is the third largest FEHB plan carrier, has released a COVID-19 vaccination equity tookit and that Geisinger, a Pennsylanvia based FEHB plan carrier, has produced a Neighborly website chock full of community resources. A speaker referenced this New York Times article on the following topic: “Half of American adults have received at least one shot of the coronavirus vaccine. Now comes the hard part: persuading the other half to get it.”
In COVID-19 vaccine news from outside the carrier conference
- The Society for Human Resource Management reports that “To encourage more widespread vaccinations, President Joe Biden has announced a paid leave tax credit to employers that provide full pay for any employee who takes time off to get a COVID-19 vaccination. The tax credit is available to organizations with fewer than 500 employees, and it also provides full pay for employees who take time to recover from the vaccination. The credit covers up to $511 per day for each vaccinated employee, and is funded by the American Rescue Plan.” The FEHBlog will post the implementing IRS notice tomorrow.
- Fierce Pharma reports that “AstraZeneca is still planning to apply for emergency use authorization of its shot in the U.S., a company spokesman confirmed. * * * If going down that road yields an endorsement from the U.S., it could help boost the damaged reputation of the shot. Much of the world, especially poorer nations, are in dire need of vaccines and global demand is expected to extend into the next few years at least. In addition, the shot has a key advantage over its mRNA rivals––its lower price, which makes it particularly attractive to developing nations.”
- Govexec reports that “State Department Spokesperson Ned Price said on Tuesday that the department had delivered vaccines to all of its posts abroad, as of Sunday.“ and “The Defense Department said on Tuesday it expects to start receiving 390,000 vaccine doses weekly, which is up from an average of 155,500 per week. “[Eighty-three] percent of vaccines received by the Defense Department have been administered, exceeding the U.S. average of 78%, and more than 28% of our total force is now vaccinated,” Pentagon Press Secretary John Kirby said during a briefing on Monday.
There was a lot of carrier conference discussion about expensive yet curative cell and gene therapies. The FEHBlog ran across this recent MIT report on that topic.
Also the FEHBlog was overjoyed to hear from an OPM speaker that with any luck laterthis decade OPM will begin providing carriers with HIPAA 820 standard transactions that will allow them to reconcile premiums to headcount. The FEHBlog has been advocating this logical step for quite a while.
In other healthcare news —
- Healthcare Dive informs us about Elizabeth Fowler’s first public address since taking the reins of the CMS Center for Medicare and Medicaid Innovation earlier this year. “‘In my view, we’re at a really critical juncture in the path to value-based care,’ Fowler said at the National Association of Accountable Care Organizations’ spring conference on Tuesday, asking stakeholders for patience as CMMI reviews paused models and outlines a path forward.”
- Saturday is the Drug Enforcement Administration’s spring edition of National Prescription Drug Take Back Day. “National Prescription Drug Take Back Day is a safe, convenient, and responsible way to dispose of unused or expired prescription drugs at locations in communities throughout the country. The October 2020 Take Back Day brought in 985,392 pounds (492.7 tons) of medication. This is the largest amount ever collected in the program’s ten years!” You can find your nearest collection site here.