Midweek update

From the Federal Employee Benefits Open Season front —
- FedWeek offers its Open Season report.
- My Federal Retires explains Open Season options available to those with Medicare coverage.
- Govexec promotes healthcare flexible savings accounts, which are only available to federal and Postal employees. The FEHBlog was surprised to learn that “less than 20% of active feds have an FSA.” The article explains the mechanics of the FSA, among other things.
In other federal employee benefits news, Reg Jones, writing in the Federal Times, tells us how to calculate federal disability retirement benefits and answers a question about survivor annuitant coverage.
In other OPM news, Govexec tells us how the OPM Director is celebrating Work and Family Month.
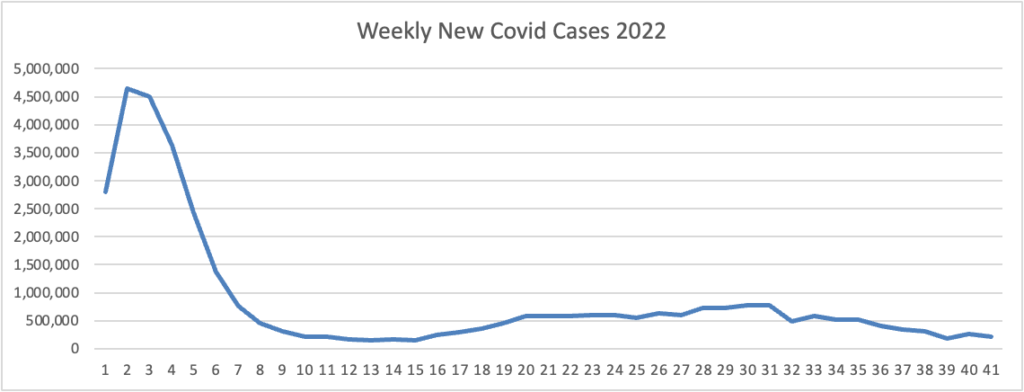
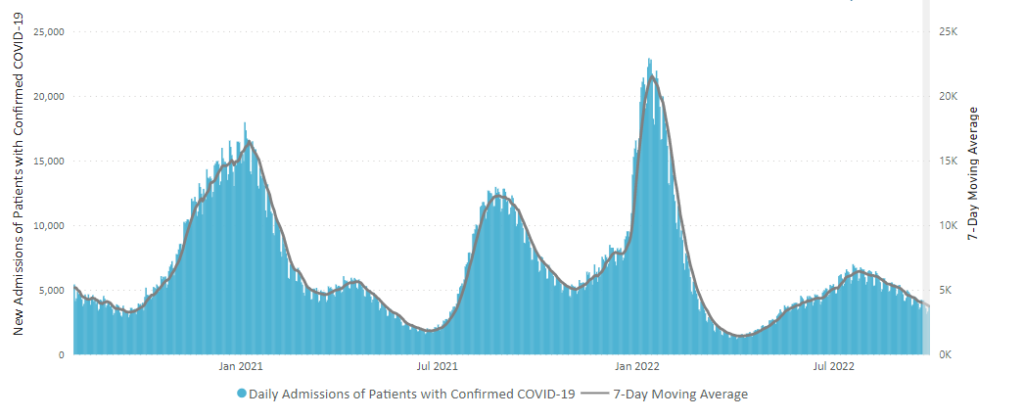
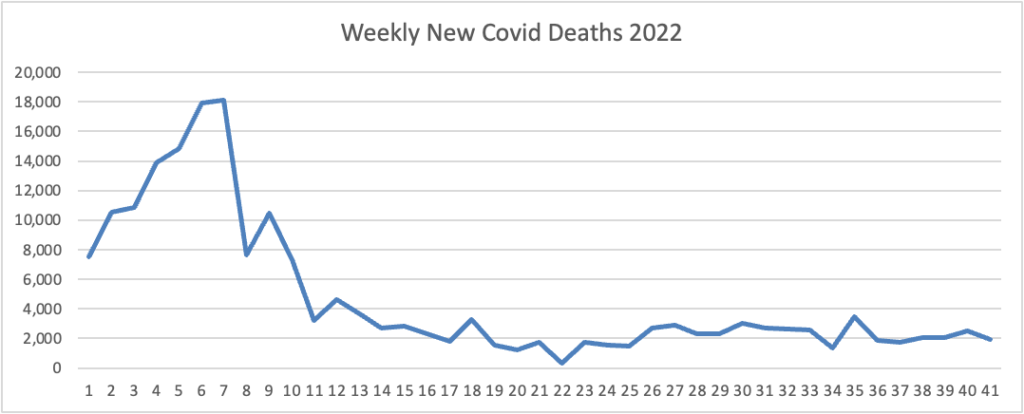
From the Omicron and siblings front, Beckers Hospital Review informs us that “Omicron subvariants BQ.1 and BQ.1.1 — dubbed “escape variants” for their immune evasiveness — are steadily gaining prevalence in the U.S. and now account for more than 16 percent of all COVID-19 cases confirmed nationwide, CDC data shows.”
Beckers adds
Data analysis from the Los Angeles-based Smidt Heart Institute at Cedars-Sinai found heart attack deaths rose significantly with COVID-19 surges, including omicron surges.
Heart attack deaths were on the decline before the pandemic. However, during COVID-19 surges, deaths increased — especially among individuals ages 25-44, according to an Oct. 24 release shared with Becker’s.
In other public health news
- The Wall Street Journal and the New York Times warn us about a potential “tripledemic” of Omicron, the flu and RSV occurring this winter.
- The New York Times also reports
A new national study has suggested that chemical hair straighteners could pose a small risk for uterine cancer. Rates of the disease are still relatively low, said Dr. Alexandra White, head of the environment and cancer epidemiology group of the National Institute of Environmental Health Sciences and the lead author on the study. The research also did not definitively show that hair straighteners cause cancer. But the findings are cause for concern, she said.
Rates of uterine cancer have been increasing in the United States, particularly for Black and Hispanic women. The number of cases diagnosed each year rose to 65,950 this year, compared to 39,000 15 years ago. Black women are also more likely to have more aggressive cases of the cancer, Dr. White said, and the study showed they were disproportionately more likely to use hair straighteners.
If you have used chemical hair straighteners, you do not need to seek out medical attention or consult your doctor unless you have symptoms for uterine cancer, said Dr. Otis Brawley, an oncologist at Johns Hopkins University. But women should regularly see a gynecologist, and be aware of the risk factors and early signs of the disease. [The article also explains uterine cancer risk factors and symptoms.]
Roll Call tells us
The Biden administration is preparing a comprehensive initiative to fight hepatitis C that would streamline testing and treatment and secure an agreement with drugmakers to bring down the cost of treatment of the disease, which has spiked during the pandemic.
Francis Collins, special project adviser to President Joe Biden and former longtime director of the National Institutes of Health, said Monday the administration hopes to secure some funding this year for the yet to be formally unveiled initiative.
He said he has briefed Biden on the plan, and the Office of Management and Budget is “enthusiastic about figuring out how to fit this into the budgetary requests.”
The National Institutes of Health announced
Long-term use of electronic cigarettes, or vaping products, can significantly impair the function of the body’s blood vessels, increasing the risk for cardiovascular disease. Additionally, the use of both e-cigarettes and regular cigarettes may cause an even greater risk than the use of either of these products alone. These findings come from two new studies supported by the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health (NIH).
From the Food and Drug Administration front —
BioPharma Dive informs us
The Food and Drug Administration on Tuesday approved a first-of-its-kind treatment for multiple myeloma from Johnson & Johnson, but put restrictions on its use due to the drug’s potentially dangerous side effects.
Healthcare providers offering the drug, which will be sold as Tecvayli, will need to follow guidelines set up in a Risk Evaluation and Mitigation Strategy, or REMS. Prescribers and pharmacies must be certified in the Tecvayli REMS program, which will focus on monitoring and counseling for patients.
The FDA has required REMS for dozens of medicines since the program was authorized by Congress in 2007. The list includes Bristol Myers Squibb’s cell therapy Abecma, which won approval for multiple myeloma last year.
Fierce Pharma relates
AstraZeneca’s long-troubled cancer immunotherapy tremelimumab has finally secured its first FDA approval, but the regulatory blessing comes in what could be an increasingly competitive tumor type.
To be sold under the brand name Imjudo, tremelimumab has won an FDA go-ahead in combination with AstraZeneca’s PD-L1 inhibitor Imfinzi for treating unresectable hepatocellular carcinoma, the most common type of liver cancer.
The FDA nod officially puts an end to the streak of clinical trial failures that tremelimumab endured over recent years in multiple cancer types, including non-small cell lung cancer, head and neck cancer and bladder cancer. But while the CTLA-4 inhibitor has now crossed the regulatory finish line, a commercial fight lies ahead.
From the Medicare front – –
- STAT News discusses a new CMS policy aimed at controlling dialysis prices.
- Fierce Healthcare tells us “Starting next year, insurers will not be able to air any television ads for Medicare Advantage (MA) plans before getting approval from federal regulators.” Tough break for Joe Namath.
From the ACA marketplace front —
- The Department of Health and Human Services discusses its plans for the upcoming Open enrollment period.
- Benefits Pro discusses the popularity of alternative health reimbursement accounts which allow employers to offer marketplace coverage to their employees.
Speaking of account-based health plans, the Plan Sponsors Council of America released its 2022 benchmarking survey of health savings accounts.
From the U.S. healthcare business front —
- Health Data Management assesses whether Amazon and Walmart can build effective value based care models.
- CVS Health released “The Rx Report: A New Day in Retail Pharmacy, highlighting the results of a CVS Health/Morning Consult survey, which identified strong consumer preference and demand for an expanded role of pharmacists.”