Midweek Update
From the Delta variant front, MedPage Today reports on a recent study supporting the FDA/CDC conclusion that all immunocompromised folks over the vaccine eligibility age should receive a COVID vaccine booster.
Kaiser Health News surveys the U.S. market for convenience COVID testing.
FedWeek reminds us that
Just days remaining before the deadline for federal employees to receive a Coronavirus vaccination under the mandate and disciplinary actions could soon follow, although indications are that such actions will not necessarily be immediate nor fast-moving.
Taking into account the two-week waiting period afterward that required to be considered fully vaccinated by the deadline of November 22, employees would need to receive either the single Johnson and Johnson vaccine or the second dose of the two-dose Pfizer or Moderna vaccines no later than next Monday (November 8).
In the third quarter financial results department, we find
Healthcare Dive reporting that
- “CVS Health’s payer business Aetna reported higher-than-expected costs for COVID-19 treatment and testing in the third quarter as the highly infectious delta variant spread and deferred care returned.
- “However, a greater volume of vaccinations and COVID-19 tests (along with pharmacy services growth) fueled a sharp jump in profit, leading the Woonsocket, Rhode Island-based company to boost its full-year outlook.
- “CVS beat Wall Street expectations on both earnings and revenue for the quarter, with a topline of $73.8 billion, up 10% year over year, contributing to net income of $1.6 billion, up 30% year over year.”
Fierce Healthcare reporting
- “Humana expects its individual Medicare Advantage membership to grow by 8% in 2022 as part of a more conservative financial outlook.
- “The insurer gave hints to its outlook for next year as part of its earnings report released Wednesday that saw Humana post a $1.5 billion profit in the third quarter but cut its financial outlook for 2021 due in part to higher-than-expected COVID-19 costs.
- “The insurer’s third-quarter earnings report, though, released Wednesday, pointed to strong growth in its Medicare Advantage offerings and lower-than-expected healthcare use among MA beneficiaries.
- “Humana also generated $20.7 billion in revenue for the third quarter, which fell short of Wall Street expectations.”
BioSpace reporting
- “Pfizer reported its third-quarter 2021 financials, citing $24.1 billion in quarterly revenues, a stunning 130% operational growth. If you exclude the sales of its COVID-19 vaccine with BioNTech, revenues grew 7% operationally to $11.1 billion. The company raised its full-year guidance to range from $81 to $82 billion.
- “’While we are proud of our third quarter financial performance, we are even more proud of what these financial results represent in terms of the positive impact we are having on human lives around the world,’ said Albert Bourla, Pfizer’s chairman and chief executive officer. ‘For example, more than 75% of the revenues we have recorded up through third-quarter 2021 for Comirnaty have come from supplying countries outside the U.S., and we remain on track to achieve our goal of delivering at least two billion doses to low- and middle-income countries by the end of 2022 — at least one billion to be delivered this year and one billion near year, with the possibility to increase those deliveries if more are placed by these countries for 2022.’”
The FEHBlog also ran across this STAT News article discussing Moderna’s unexpected approach to breaking into the CRISPR gene editing market.
Moderna, flush with cash thanks to its blockbuster Covid-19 vaccine, made waves back in August when CEO Stéphane Bancel declared the company’s next frontier to be genome editing, the nascent science of rewriting DNA to treat disease. Three months later, Moderna has picked a partner for its foray into CRISPR, and it’s not one of the field’s multibillion-dollar players.
Metagenomi, a three-year-old startup out of the University of California, Berkeley, signed a deal to help Moderna discover and develop genome-editing therapies, the companies said Tuesday. The agreement includes an up-front cash payment to Metagenomi, bonuses for meeting development milestones, and royalties on any products that arise from the collaboration. Moderna will also make an equity investment in the company. Neither side disclosed financial details.
The idea behind Metagenomi is mining nature’s infinite complexity in search of new ways to edit DNA. That means scouring the natural world for soils, sediments, and microbiomes, putting the samples through intensive genome sequencing, and sifting the results for better genome-editing mousetraps. It’s a process called metagenomics, according to company co-founder and CEO Brian Thomas, and it has already produced results.
Many genome-editing efforts rely on pairing CRISPR with an enzyme called Cas9, which functions as the molecular pair of scissors that makes cuts to DNA. By studying nature, Metagenomi has discovered newer and potentially more potent enzymes that might broaden CRISPR’s medicinal promise.
Cool.
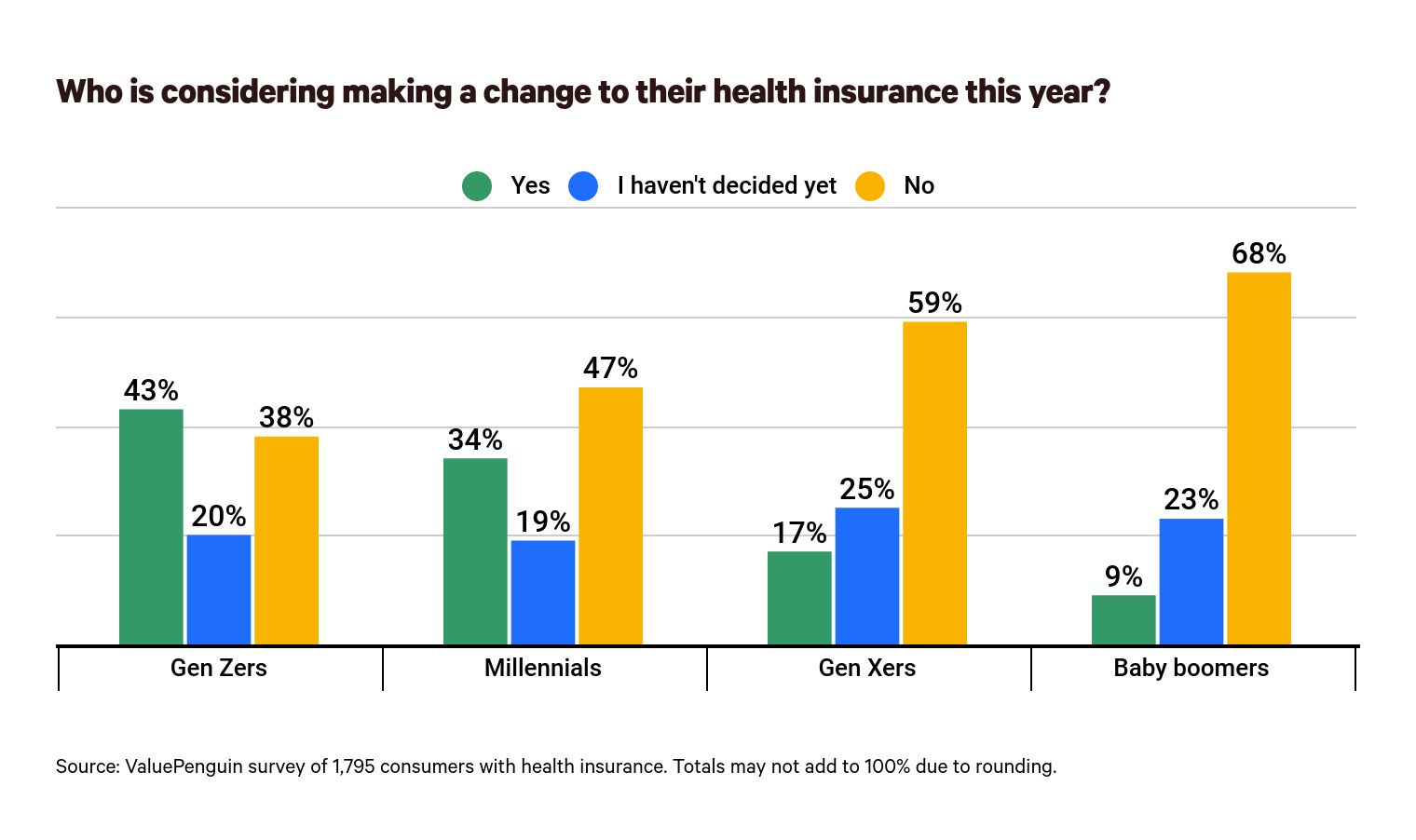
From the Open Season front, Health Payer Intelligence directed the FEHBlog to this fascinating ValuePenguin study of consumer attitudes toward health benefit open seasons. For example, eagerness to consider changing plans steadily decreases with age which may explain the relatively low turnover rate that typically occurs in FEHB Open Seasons.
EHR Intelligence reports that the federal government is putting its weight behind the adoption of The HL7 Gender Harmony Logical Model in U.S. electronic health record systems:
- “Gender identity (GI): an individual’s personal sense of being a man, woman, boy, girl, or something else.
- “Sex for clinical use: a summary sex classification element based on clinical observations like organ survey, hormone levels, and/or chromosomal analysis.
- “Recorded sex or gender (RSG): sex values or gender values that are specified administrative documents such as identity cards or insurance cards.
- “Name to use (NtU): the name that the patient wishes to use in healthcare interactions.
- “Pronouns: the English language third-person personal pronoun determined by the patient for use in healthcare interactions, clinical notes, and written instructions to caregivers.”
Assuming the model works with EHR system, it would be a logical next step to extended this model to health plan claim and customer service systems.