Midweek Update

From the Omicron and siblings front
The Wall Street Journal reports
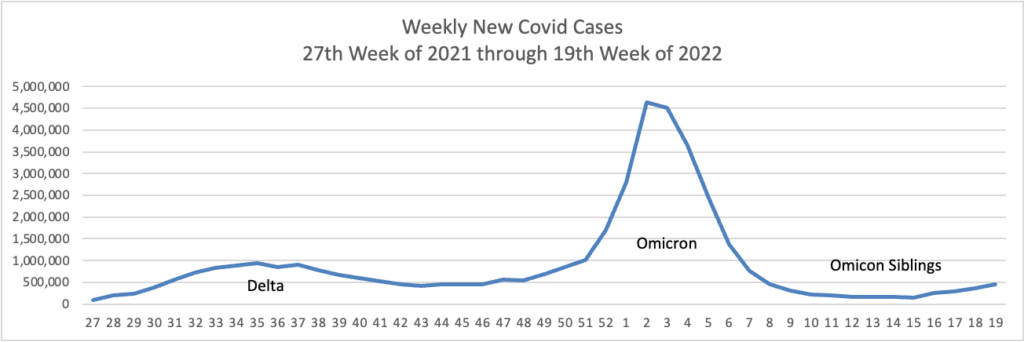
The seven-day moving average of new Covid-19 cases recently topped 94,000 a day, Centers for Disease Control and Prevention data show, nearly four times lows reached in late March. The true number of new cases is likely significantly higher, epidemiologists say, because so many people are self-testing at home or not testing at all.
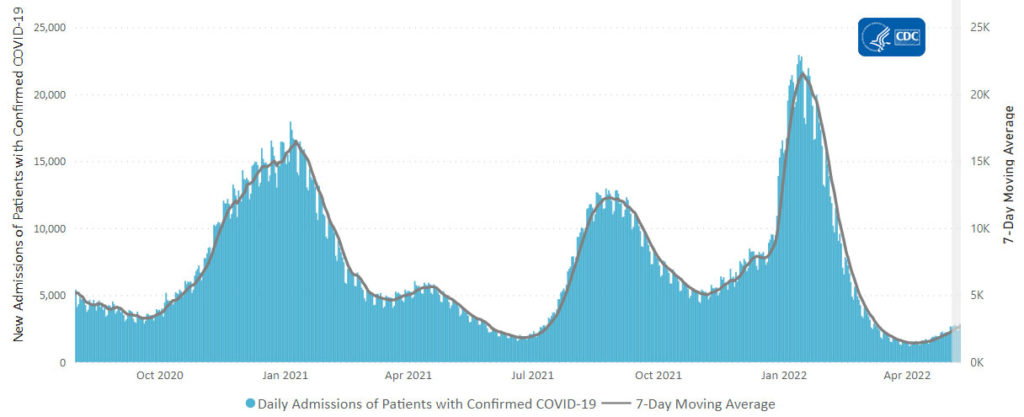
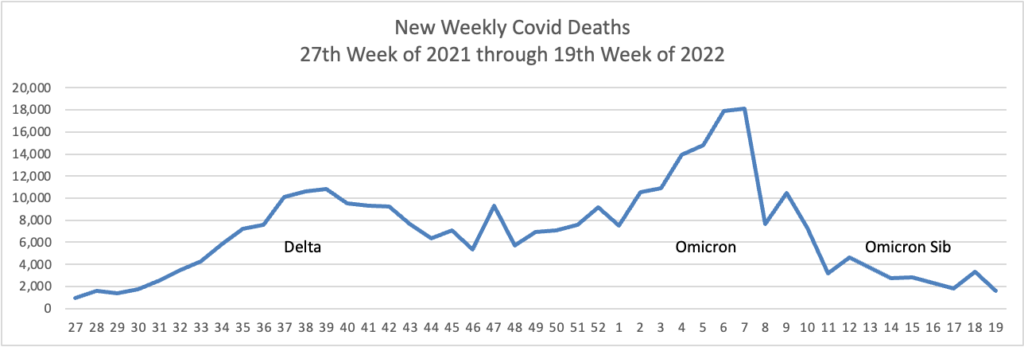
The rise in cases hasn’t translated thus far into major surges in severe illness. The seven-day average of confirmed cases in hospitalized patients reached about 18,550 on Wednesday, up from lows near 10,000 in mid-April, but far below a record peak above 150,000 in January. The numbers include people who test positive on routine screening after getting hospitalized for other reasons. The daily average of reported deaths has slipped under 300 a day, the lowest point since last summer.
But * * * the more an outbreak spreads, the more likely it will reach the most vulnerable including elderly people and others with compromised immune systems, the experts say, and the more likely the virus will continue to mutate.
Bloomberg Prognosis adds
As Covid-19 again surges across the US, many people are going without time-sensitive therapeutics like Paxlovid because doctors worried about shortages are reluctant to prescribe the drugs. But the situation has changed and supplies are now abundant.
The Food and Drug Administration has issued emergency-use authorizations for the drug to treat mild to moderate Covid-19 in people who are at high risk. The Centers for Disease Control and Prevention defines those as individuals ages 50 years or older, unvaccinated, or with certain medical conditions like kidney, liver, lung and heart disease, diabetes, cancer and HIV. It also recommends the drug for people who are immunocompromised, pregnant, obese, cigarette smokers or suffering from mood disorders.
You can find the one stop test to treat locations “by using the Department of Health and Human Services’ Test to Treat Locator or by calling 1-800-232-0233.”
Kaiser Health News recommends “improving ventilation and filtration of the air. ‘Ventilation matters a lot,’ said Dr. Amy Barczak, an assistant professor of medicine at Harvard Medical School. ‘If you’re taking care of someone at home, it’s really important to maximize all the interventions that work.’”
Viral particles float through the air like invisible secondhand smoke, diffusing as they travel. Outside the home, viruses are quickly dispersed by the wind. Inside, germs can build up, like clouds of thick cigarette smoke, increasing the risk of inhaling the virus.
The best strategy for avoiding the virus is to make your indoor environment as much like the outdoors as possible.
In related viral news, Beckers Hospital Review tells us
More than 400 children worldwide have developed unusual cases of acute hepatitis, and researchers are still searching for the cause of the outbreak, the World Health Organization said May 17.
As of May 15, the WHO reported 429 probable cases in 22 countries, up from 348 cases a week prior, according to Philippa Easterbrook, MD, a senior scientist in the global hepatitis program at the WHO. Another 40 cases are still under investigation, and 75 percent of all affected children are under age 5.
Twelve countries are reporting more than five cases, double the amount from last week. Of these 12 countries, nine are in Europe. In total, six children have died in the outbreak and 26 have required liver transplants, according to Dr. Easterbrook.
As of May 17, researchers were still investigating the cause of the hepatitis outbreak. The leading hypothesis is that an adenovirus and SARS-CoV-2, the virus that causes COVID-19, may be causing hepatitis in children. Scientists are exploring “how these two infections may be working together as co-factors either by enhancing susceptibility or creating an abnormal response,” Dr. Easterbrook said.
From the healthcare policy front, AHIP today launched
Healthier People through Healthier Markets, a new policy roadmap and set of solutions to improve health care affordability and access for every American. The effort is focused on boosting competition in health care markets and reining in harmful practices that hurt American families. With the launch of this policy roadmap, AHIP sent letters to President Biden and the leadership of Congress that lay out a detailed set of legislative and regulatory enforcement actions to increase competition in health care, drive down costs, and improve health care access for patients.
The FEHBlog supports this approach.
From the mental healthcare front, Govexec reports
The Office of Personnel Management on Wednesday urged federal agencies to ensure their employees are aware and can access the mental health benefits provided to federal workers, in light of May being Mental Health Awareness Month.
In a memo to agency heads, OPM Director Kiran Ahuja noted that promoting the federal workforce’s wellbeing, including mental health, is a priority in President Biden’s management agenda.
“We want to make sure that all federal employees understand the supports available to them and underscore that there should be no shame or stigma for taking care of their mental health,” Ahuja wrote. “[As] a reminder, employee assistance programs and Federal Employees Health Benefits health plans offer mental health services to employees and their family members. We encourage agencies to proactively communicate to their workforces about their options and encourage employees to contact their agency benefits officers or EAP coordinator to learn more.”
The FEHBlog encourages OPM to better coordinate mental health care services among FEHB plans, EAPs and wellness programs.
From the telehealth front
- NCQA has released a new whitepaper covering two of health care’s intersecting priorities. The full text of the white paper, titled The Future of Telehealth Roundtable: The Potential Impact of Emerging Technologies on Health Equity, is available on the NCQA website. Check it out.
- mHealth Intelligence informs us “In the second half of 2020, only 14.1 percent of children used telehealth due to the pandemic, but use was higher among those with asthma, a developmental condition, or a disability, the Centers for Disease Control and Prevention (CDC) found.”
From the survey department, Beckers Payer Issues advises that “Castlight Health analyzed more than 160 million commercial medical claims nationwide to reveal insights about healthcare utilization patterns from 2018 to 2021.” Castlights report ranks the fifty States and DC based on average medical spending per member in 2021.
From the miscellany department —
- Beckers Payer Issues reports “Anthem shareholders voted at their annual meeting May 18 to change the company’s name to Elevance Health.”
- Federal News Network discusses the Postmaster General’s plans to close and consolidate Postal facilities across the delivery network. “The network transformation initiative will impact nearly 500 network mail processing locations, 1,000 transfer hubs and 100,000 carrier routes. It will also impact 10,000 delivery units, which USPS defines as post offices, stations, branches or carrier annexes that handle mail delivery functions.”
- FedSmith tells us “Starting May 26, 2022, federal retirees will notice a new process for signing into the OPM Retirement Services Online website. The login process will now be managed through the federal government’s Login.gov website and will require you to create a new username and password at login.gov if you do not currently have one.”