Thursday Miscellany

From our Nation’s capital, the New York Times reports,
White House officials said on Wednesday that they would have to repurpose federal Covid-19 funds meant for coronavirus tests and protective equipment in order to supply more antiviral pills and vaccines, after so far failing to persuade Congress to pass a new pandemic relief package.
Roughly $10 billion from Department of Health and Human Services funds will be rerouted, around half of it to purchase vaccines for Americans ahead of a possible fall or winter wave of virus cases, when an updated shot may be needed, according to one White House official. The other half will go mostly to purchasing 10 million courses of Paxlovid, the antiviral treatment made by Pfizer that has been shown to substantially reduce the severity of Covid-19 in high-risk people, the official said. Around $300 million will be spent on another kind of treatment, monoclonal antibodies.
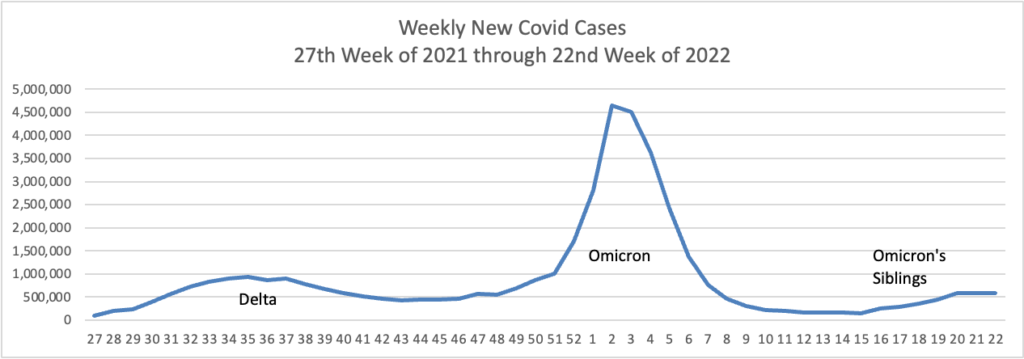
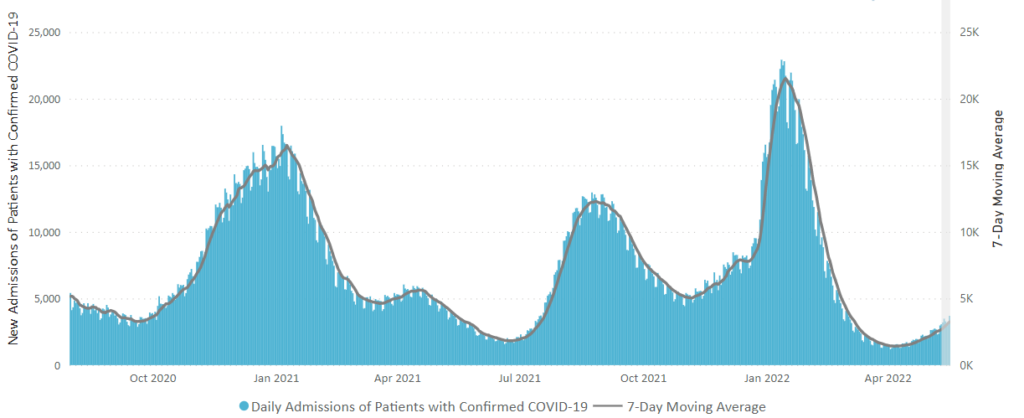
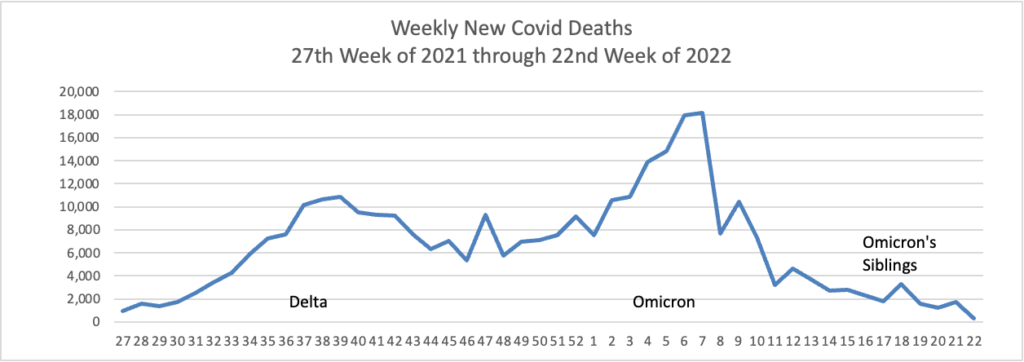
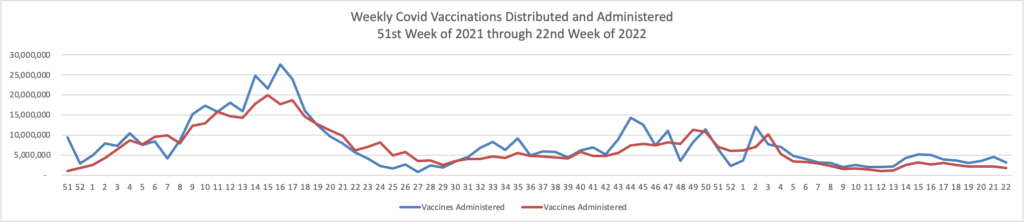
Also from the Omnicron and siblings front, a friend of the FEHBlog, journalist Theresa Defino, points out
Today and tomorrow NIH’s Advisory Committee to the Director is holding its first of two annual meetings. Today Dr. Fauci gave a presentation on Covid and Dr. Walter Koroshetz, director of the National Institute of Neurological Disorders and Stroke, spoke on recovery from Covid.
The most interesting comments Dr. Fauci made begin on page 45. Dr. Koroshetz’s talk was about NIH’s efforts to understand long COVID. Lots of trials are going on. He also mentioned this website on Covid recovery which is worth a look.
From the unusual viruses report, Becker’s Hospital Review brings us up to date on roughly 700 cases of acute hepatitis of unknown etiology infecting young children in 34 countries, including our own. “The U.S. has reported 274 probable hepatitis cases in 39 states and jurisdictions as of June 8, according to the CDC.”
From the maternal health front, the American Hospital Association informs us
The Health Resources and Services Administration has released a report evaluating the Rural Maternity and Obstetrics Management Strategies Program, which completed its first year last August. The program uses a network approach to coordinate and improve maternal health care from preconception to postpartum; telehealth services to increase access to care in rural areas; potential aggregation of low-volume rural obstetric services; and payment structures that promote financial sustainability for access to high-quality maternal care. The cohort includes networks in Missouri, New Mexico and Texas that provided prenatal, delivery and postpartum care to 3,101 rural mothers. Participants said hiring patient navigators emerged as an early success strategy. The networks also laid the groundwork for expanding telehealth.
From the Rx coverage front, STAT News offers an interesting article about the drug pricing reform debate ongoing in Congress using an AMA Journal report showing skyrocketing launch prices for newly approved drugs.
Health Payer Intelligence tells us that “AHIP has subscribed to the Institute for Clinical and Economic Review’s (ICER) cloud-based analytics platform, providing [its] health plan members with access to benchmark reports, cost-effectiveness data, and policy recommendations.” Good idea, AHIP.
From the federal employee benefits front, a financial planner discusses how divorce may affect FEHB and FEGLI benefits at the My Federal Retirement website.
From the HIPAA standard transactions front, the CMS National Standards Group has released an updated Compliance Review Program Findings report identifying the most common violations of those standard and operating rules from compliance reviews.