Friday Stats and More
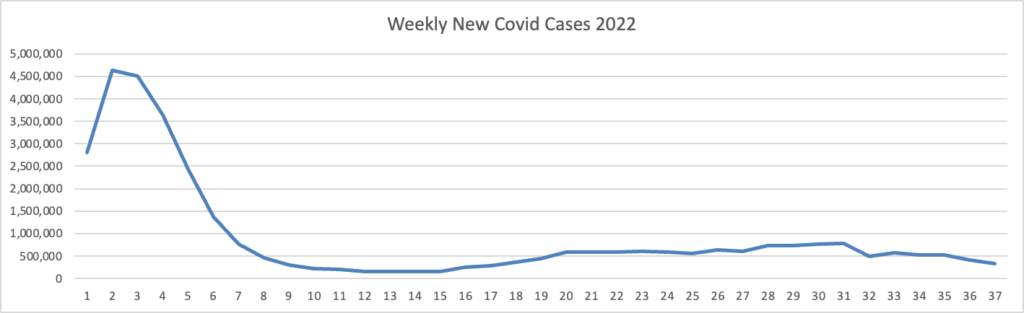
Based on the Centers for Disease Control’s Covid Data Tracker and using Thursday as the first day of the week, here is the FEHBlog’s latest chart of weekly new Covid cases 2022:
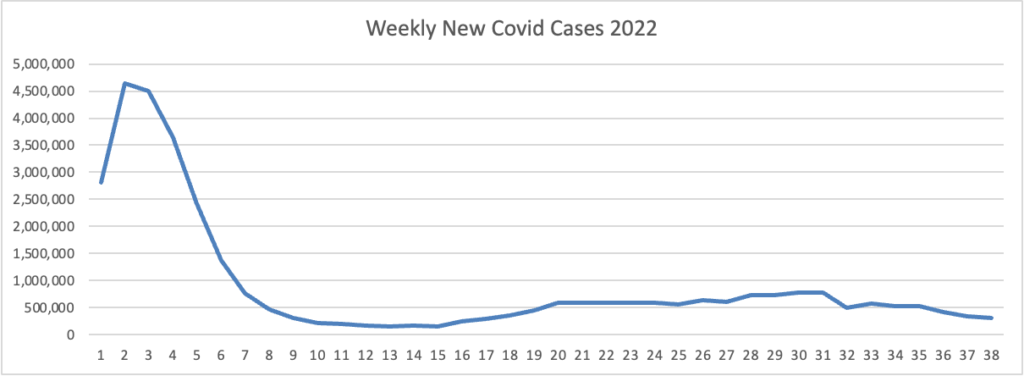
The bulge on the left side of the chart is the original Omicron. The CDC’s weekly interpretation of its Covid stats adds
As of September 21, 2022, the current 7-day moving average of daily new cases (54,186) decreased 10.6% compared with the previous 7-day moving average (60,593).
CDC Nowcast projections* for the week ending September 24, 2022, estimate that the combined national proportion of lineages designated as Omicron will continue to be 100%. There are five lineages designated as Omicron: BA.5, BA.4.6, BA.4, BF.7, and BA.2.75. The predominant Omicron lineage is BA.5, projected at 83.1% (95% PI 81.3-84.7%).
The New York Times asks
Where is Pi?
Last year, the World Health Organization began assigning Greek letters to worrying new variants of the coronavirus. The organization started with Alpha and swiftly worked its way through the Greek alphabet in the months that followed. When Omicron arrived in November, it was the 13th named variant in less than a year.
But 10 months have passed since Omicron’s debut, and the next letter in line, Pi, has yet to arrive.
That does not mean SARS-CoV-2, the coronavirus that causes Covid-19, has stopped evolving. But it may have entered a new stage. Last year, more than a dozen ordinary viruses independently transformed into major new public health threats. But now, all of the virus’s most significant variations are descending from a single lineage: Omicron.
“Based on what’s being detected at the moment, it’s looking like future SARS-CoV-2 will evolve from Omicron,” said David Robertson, a virologist at the University of Glasgow.
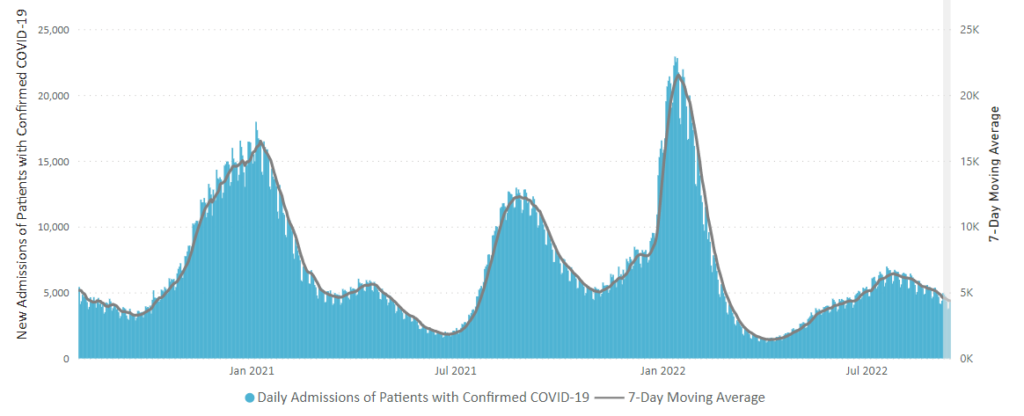
Here is the CDC’s latest chart of daily new Covid hospitalization trends:
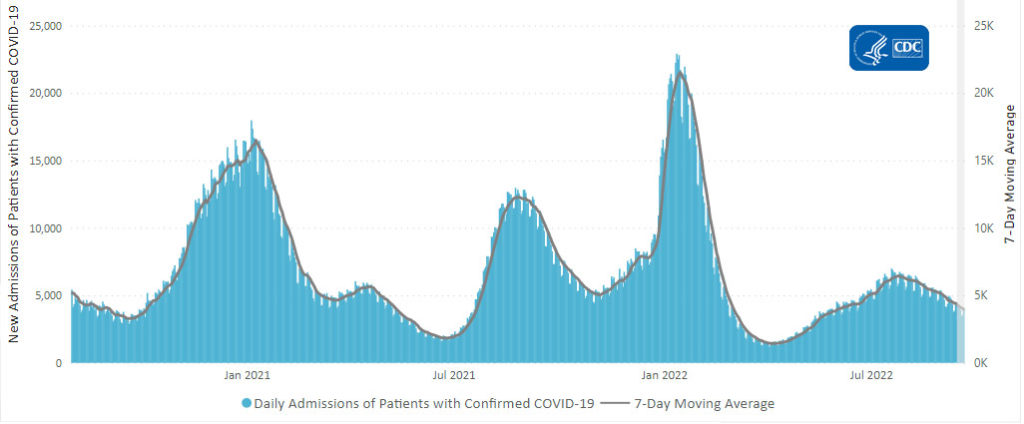
The weekly CDC review adds
The current 7-day daily average for September 14–20, 2022, was 3,971. This is a 9.9% decrease from the prior 7-day average (4,410) from September 7–13, 2022.
CDC’s Coronavirus Disease 2019-Associated Hospitalization Surveillance Network (COVID-NET) shows that COVID-19-associated hospitalizations continue to affect adults ages 65 years and older. Since early April 2022, more than 50% of all COVID-19-associated hospitalizations occurring every week are among adults ages 65 years and older. Before April 2022, adults ages 65 years and older had not comprised more than half of all COVID-19-associated hospitalizations since January 2021.
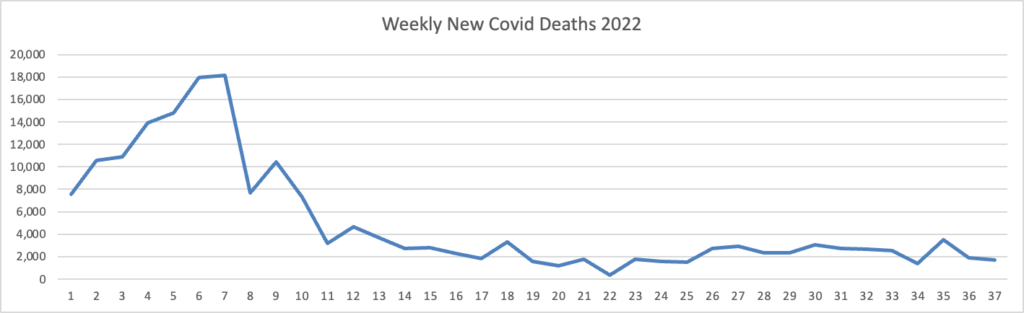
Here’s the FEHBlog latest chart of new weekly Covid deaths
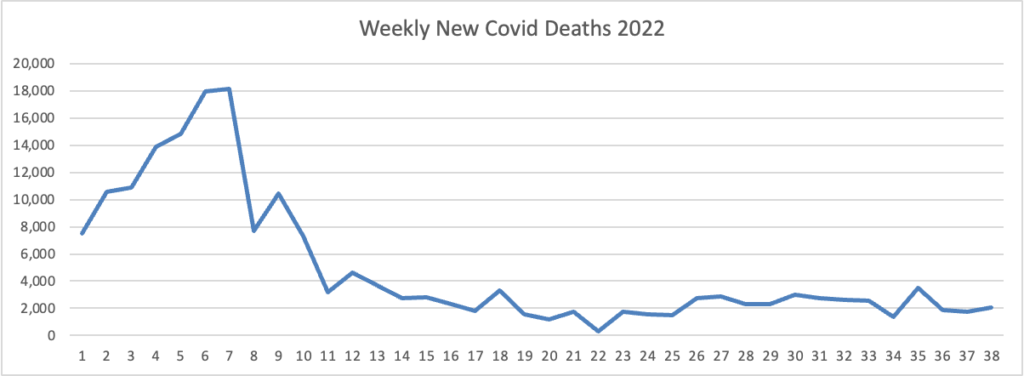
The weekly CDC review adds “The current 7-day moving average of new deaths (347) decreased 12.2% compared with the previous 7-day moving average (396).”
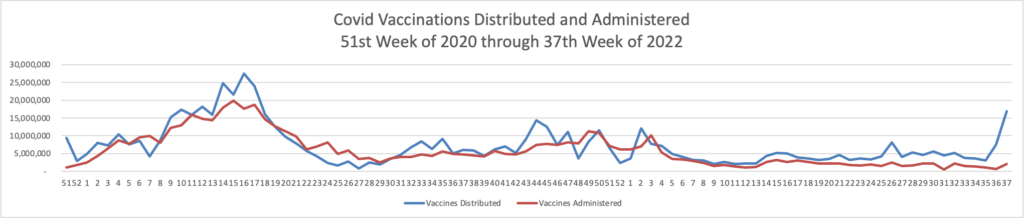
Here is the FEHBlog’s chart of Covid vaccinations distributed and administered from the beginning of the Covid vaccination era, the 51st week of 2020, and the recently end 38th week of 2022.
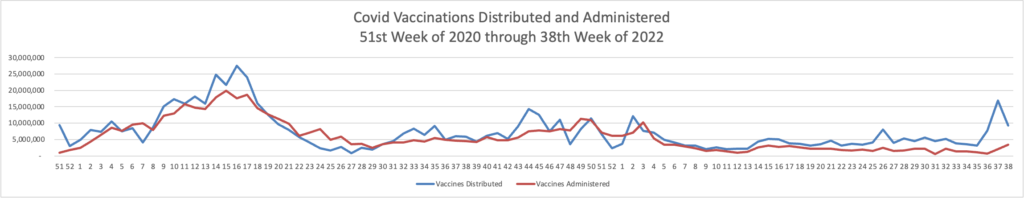
The weekly CDC review adds
As of September 21, 2022, 616.2 million vaccine doses have been administered in the United States. Overall, about 263.8 million people, or 79.5% of the total U.S. population, have received at least one dose of vaccine. About 225.0 million people, or 67.8% of the total U.S. population, have completed a primary series.
Of those who have completed a primary series, about 109.6 million people have received a booster dose,* and 4.4 million people have received an updated (bivalent) booster dose. But 49.9% of the total booster-eligible population has not yet received a booster dose. Booster dose eligibility varies by age and health condition. Learn more about who is eligible.
It’s worth noting that according to the CDC’s Covid Data Tracker 92.3% of Americans 65 and older have received the first two vaccination doses; 71% of this cadre as received one booster dose, and 43% of this cadre, including the FEHBlog, has received two booster doses. Given the COVID-NET news above, these are the most important statistics.
In CDC Communities Level news, the weekly CDC review points out
As of September 22, 2022, there are 226 (7.0%) counties, districts, or territories with a high COVID-19 Community Level, 1,005 (31.2%) counties with a medium Community Level, and 1,986 (61.7%) counties with a low Community Level. Compared with last week, this represents a large decrease (−6.3 percentage points) in the number of high-level counties, a moderate decrease (-4.7 percentage points) in the number of medium-level counties, and a large increase (+11.0 percentage points) in the number of low-level counties.
In other virus news, the New York Times reports
With monkeypox cases on the decline nationally, federal health officials expressed optimism on Thursday that the virus could be eliminated in the United States, though they cautioned that unless it was wiped out globally, Americans would remain at risk.
“Our goal is to eradicate; that’s what we’re working toward,” Dr. Demetre Daskalakis, the deputy coordinator of the White House monkeypox response team, said during a visit to a monkeypox vaccination clinic in Washington. He added, “The prediction is, we’re going to get very close.”
From the Rx coverage front, EndPoint News informs us
Drug pricing experts generally agree that bluebird bio’s two recently approved gene therapies and their multimillion-dollar price tags aren’t going to be one-offs as a wave of new cell and gene therapies makes its way to the market.
The FDA’s recent approvals for bluebird’s $2.8 million Zynteglo — with ICER supporting the price and an 80% rebate if patients don’t achieve transfusion independence — and the $3 million Skysona, approved under accelerated approval, are likely to be the norm for gene therapy prices moving forward, particularly if they can reduce costs elsewhere in the health care ecosystem, experts said.
Daniel Ollendorf, director of value measurement & global health initiatives at the Center for the Evaluation of Value and Risk in Health at Tufts Medical Center, told Endpoints News in a phone interview that the trend behind multimillion-plus gene therapies is an extension of what began with Novartis’ $2.1 million spinal muscular atrophy gene therapy Zolgensma, which is still priced at about half of the 10-year current cost of chronic SMA therapy, and became a blockbuster for Novartis last year with more than $1.35 billion in annual sales.
But the expectation is that these high list prices will come with risk-sharing agreements and refunds if the products don’t work so payers don’t have to bear the full brunt of the financial risk, Ollendorf said. And he noted that some gene therapies don’t lend themselves as well to tracking milestones, but that isn’t the case for observing transfusion independence in those receiving Zynteglo.
From the maternity care front, Health Payer Intelligence tells us
Payers are implementing new programs that capitalize on telehealth and partnerships with technology companies to better engage pregnant members and improve maternal health outcomes. * * *
AHIP encouraged payers to integrate health technologies like telehealth into perinatal and maternal health to close access gaps to obstetric care in rural and underserved communities.
Perinatal telehealth interventions to improve outcomes can include videoconferences to replace or supplement in-person visits and enable consultation with specialists remotely, AHIP mentioned.
In the postpartum period, telehealth and other tools can be implemented to drive earlier postpartum follow-up visits and provide access to lactation consultants (tele-lactation).
Within the last year, Capital District Physicians’ Health Plan and Harvard Pilgrim Health Care partnered with digital family health platform Ovia to help members navigate fertility, pregnancy, and early parenting.
Through this partnership, members will gain access to three mobile apps – Ovia Fertility, Ovia Pregnancy, and Ovia Parenting in an effort to reduce maternity costs and improve maternal outcomes such as lowering c-section rates, preterm delivery, and neonatal intensive care unit (NICU) stays.
From the human interest front, Forbes explains “Why Billionaire Eric Schmidt Is Backing A High School Senior Making A Cancer-Detecting Toothbrush And Other Brilliant Teens.”