Thursday Miscellany

The FEHBlog enjoyed watching a love-fest of a Congressional business meeting this morning when the House Oversight and Reform Committee approved the Postal Reform Act, HR 3076, by a voice vote. Govexec reports on the proceedings and adds that the Senate has confirmed two Postal Service Board of Governors nominees. OPM Director nominee Kiran Ahuja had her confirmation hearing on the same day as these new Postal Governors. Her nomination should be considered on the Senate floor soon.
From the COVID-19 front
- Bloomberg reports that ““Anyone who is fully vaccinated can participate in indoor or outdoor activities, large or small, without wearing a mask or physical distancing,” said CDC Director Rochelle Walensky. “If you are fully vaccinated [two weeks after two doses], you can start doing the things that you had stopped doing because of the pandemic. We have all longed for this moment when we can get back to some sense of normalcy.” Of course, “the CDC guidance spelled out ample exceptions, however, that signal the era of masks isn’t over yet. The agency still recommends fully vaccinated people wear masks on “all planes, buses, trains and other forms of public transportation,” as well as in health care settings, correctional facilities, homeless shelters, and where required by state and local governments, or businesses.”
- Fierce Healthcare informs us that large pharmacy chains such as CVS Health, Rite Aide and Walgreen’s, are scheduling Pfizer vaccine appointments for young adolescents ages 12-15 following issuance of FDA and CDC approval over the last week.
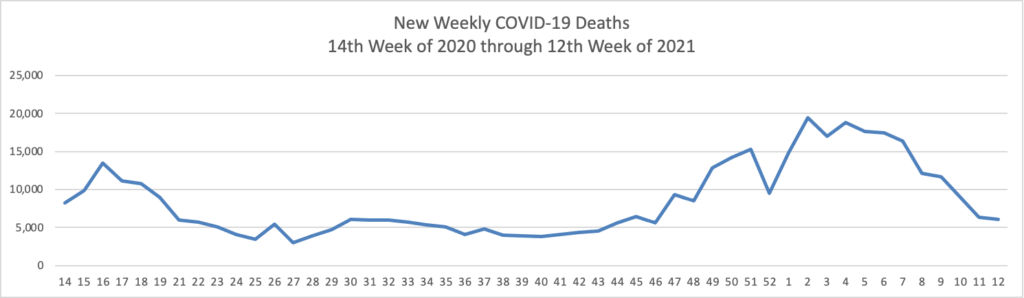
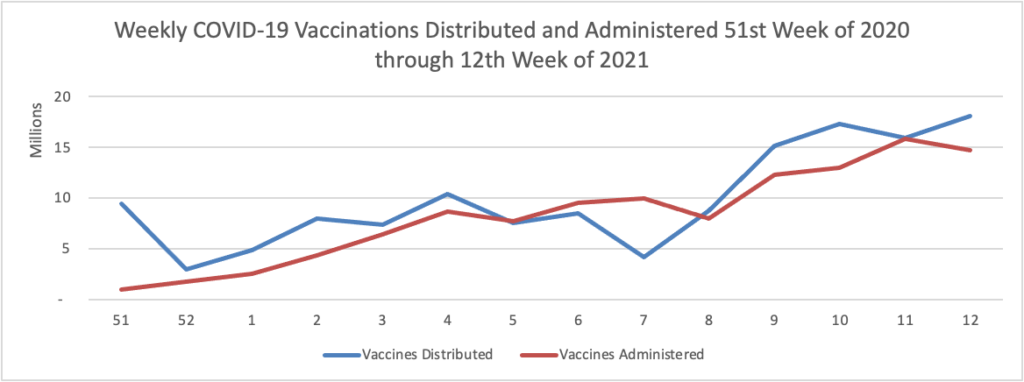
- Kaiser Health News reports that “Hispanics who have yet to receive a covid shot are about twice as likely as non-Hispanic whites or Blacks to say they’d like to get vaccinated as soon as possible, according to a survey released Thursday. The findings hint at fixable, though difficult, vaccine access problems for the population. One-third of unvaccinated Hispanics say they want the shots, compared with 17% of Blacks and 16% of whites, according to the survey released Thursday by KFF.” As of today, 59% of Americans over age 18 have received at least one dose of a COVID vaccine.
- The Wall Street Journal reports that “Prominent scientists are calling for a deeper investigation into the origin of Covid-19, including the possibility that a laboratory accident released the new coronavirus that caused the pandemic. In a letter published Thursday in the journal Science, an international group of 18 biologists, immunologists and other scientists criticized the findings of a report released in March by a World Health Organization-led team into the pandemic’s origin and called for a more extensive evaluation of the two leading hypotheses: that the pandemic virus entered the human population and began spreading after escaping from a lab or after jumping to humans from infected animals.”
- Govexec tells us that “The OPM Office of the Inspector General published a report analyzing the agency’s response to the coronavirus pandemic, finding officials fell short in a number of areas. In addition to failing to adequately inform employees of COVID-19 “incidents,” the agency failed to adequately document post-incident workplace cleaning or cleaning of “high contact” areas of its Washington, D.C., headquarters. The inspector general also said the agency needed more signage regarding social distancing and other ways to mitigate spread of the disease.” Who hasn’t fallen short at some point during these extraordinary circumstances? (Fortune Magazine suggests that New Zealand Prime Minister Jacinda Adern may be the exception that proves the rule.)
In other public health news,
- The Centers for Disease Control offers the public a pre-diabetes risk test.
- Health Payer Intelligence reports that “In response to the coronavirus pandemic’s influence on moms’ and caregivers’ stress levels, CVS Health and Aetna are taking steps to prioritize the mental well-being of individuals in these groups.” Bravo.
In healthcare business news, Healthcare Dive informs us that
- Telehealth giant Amwell saw a rise in revenue and visits in the first quarter, but its growth is decelerating from 2020, bolstering market fears about the sustainability of the virtual care boom.
- In quarterly results released aftermarket Wednesday, the Boston-based telehealth vendor beat Wall Street expectations on earnings but missed on revenue. Its topline was $57.6 million, up 7% year over year, spurred by subscription and digital revenue growth. In comparison, Amwell notched 34% year-over-year growth in the fourth quarter.
- Similarly, Amwell’s total visits of 1.6 million were up 121% year over year, paling in comparison to the 351% growth seen in the fourth quarter.
Also from Healthcare Dive
- Kaiser Permanente and Mayo Clinic are investing $100 million in a hospital-at-home company as the COVID-19 pandemic accelerates the push toward care settings outside a hospital’s four walls.
- The investment is in Boston-based Medically Home, which has a virtual and physical delivery model allowing providers to shift acute care typically administered in a hospital to a patient’s home. Its software platform, called Cesia Continuum, integrates communications and monitoring for care teams.
- The partnership will allow patients to be treated at home for infusions and conditions like cancer, infections and COVID-19, according to the companies’ announcement Thursday.