Thursday Miscellany

From the Capitol Hill front, today, the Senate took the following action
PN1166: Krista Anne Boyd, of Florida, to be Inspector General, Office of Personnel Management– Considered by Senate.– Confirmed by the Senate by Voice Vote.
And just like that, OPM has a Senate-confirmed Inspector General for the first time in over six years. The FEHBlog wishes Inspector General Boyd good luck.
The President issued a statement on World Health Day, which was celebrated today.
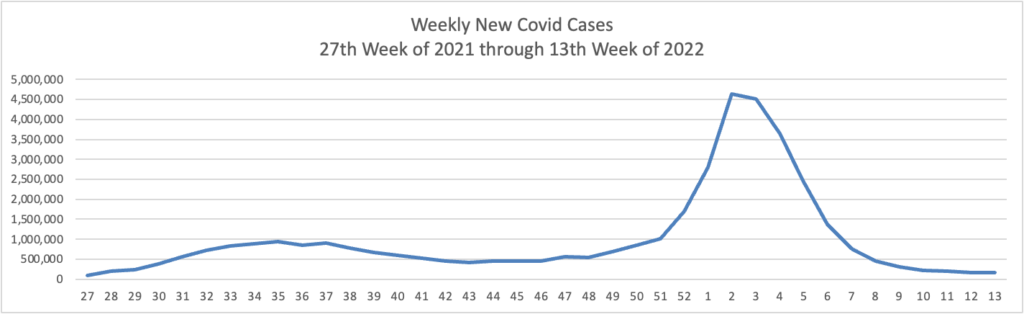
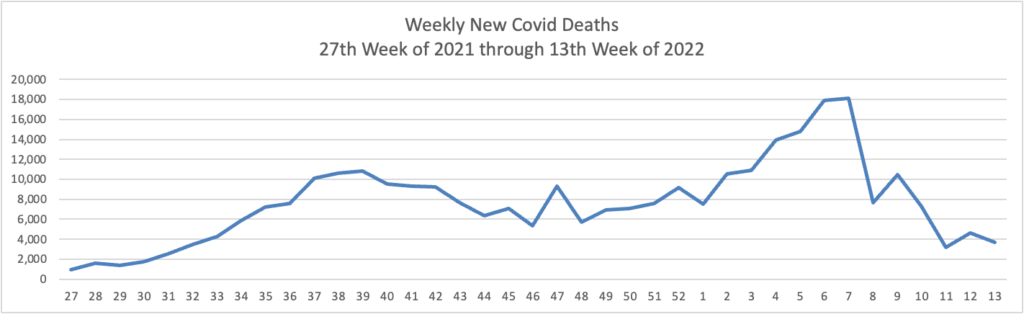
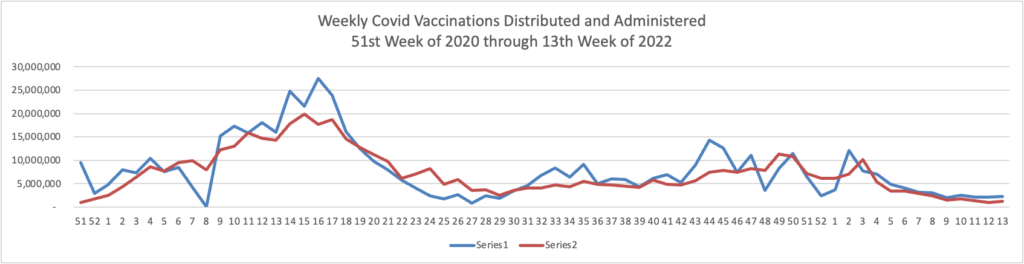
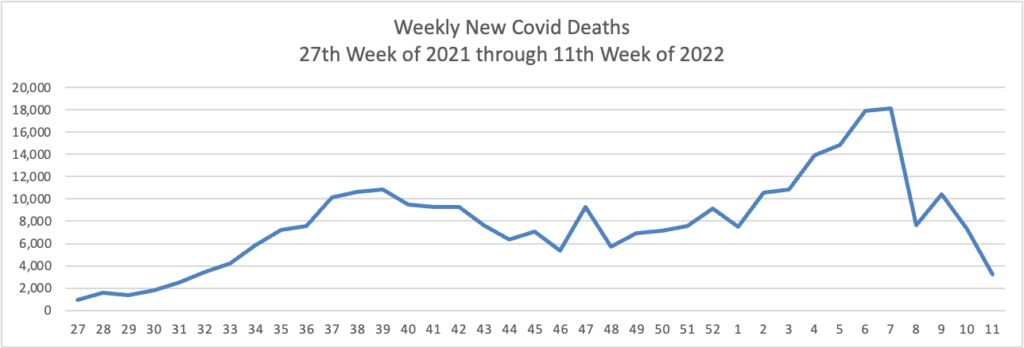
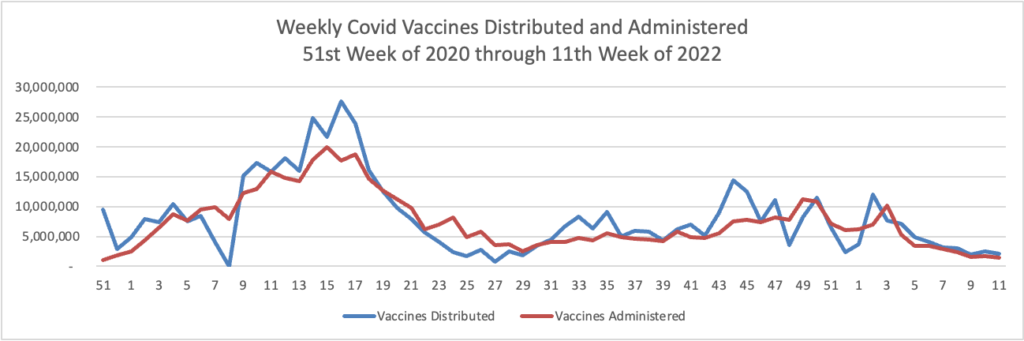
From the Omicron and siblings front —
- The Centers for Medicare Services released an updated Toolkit on Covid Vaccines for health insurers and Medicare Advantage plans.
- Govexec reports
A federal appeals court has reinstated President Biden’s COVID-19 vaccine mandate for the federal workforce, overturning a lower court’s nationwide pause that had been in effect since January.
The plaintiffs who brought their suit over Biden’s executive order did not have standing in the federal circuit, a panel of the U.S Court of Appeals for the Fifth Circuit said in a 2-1 opinion Thursday evening, and instead must pursue their appeals through the Merit Systems Protection Board or Office of Special Counsel as laid out in the Civil Service Reform Act. The court vacated the injunction and instructed the district court in Texas that issued it to dismiss the case upon remand.
From the Rx coverage front —
STAT News reports
Medicare on Thursday finalized its plan to restrict coverage for the controversial, pricey Alzheimer’s drug Aduhelm to patients participating in clinical trials.
The decision marks the end of an intense pressure campaign from drugmakers and some patient groups who wanted Medicare to reverse its initial proposal and pay for the drug for more patients. As clinical trials are usually run out of major medical centers, the decision will likely mean some interested patients won’t be able to access the drug. However, Medicare isn’t explicitly requiring that patients be treated at hospital-based clinics like the initial proposal.
The decision has implications beyond Aduhelm’s manufacturer, Biogen, as well. The coverage decision is not specific to Aduhelm, and applies to all drugs in the class, including a forthcoming treatment that Eli Lilly has begun to submit for FDA approval.
But in a major change from the initial proposal, Medicare officials created a sort of shortcut path for drugs that, unlike Aduhelm, demonstrate a clinical benefit for patients before they are approved. Medicare will cover those medicines for a broader group of patients.
They would still need to collect some data, but the possible design of the studies is much more flexible — a significant win for Lilly.
Here is a link to the CMS fact sheet on this decision.
U.S. News adds “Medicare said Thursday it’s considering a cut in enrollee premiums after officials stuck with an earlier decision to sharply limit coverage for a pricey new Alzheimer’s drug projected to drive up program costs.” Given Medicare’s shaky financial condition, one would expect the government to build up reserves with the additional cash and then adjust the premium for the following Medicare year, taking all considerations into account.
From the No Surprises Act front, AHIP released a new resource reflecting on the first 100 days of the NSA.
From the healthcare business front, Fierce Healthcare informs us
[Blue Cross of California and Walgreen] are launching new Health Corners in 12 Walgreens stores in the San Francisco Bay area and Los Angeles County.
At the Health Corner locations, Blue Shield members and customers will be able to connect with health advisers who can offer simple in-store care as well as assistance with preventive screenings, chronic care management and medications. The health advisers have clinical backgrounds, such as pharmacists or nurse practitioners.
The partnership seemed a natural fit, D.D. Johnice, vice president of the Health Transformation Lab at Blue Shield of California, said in an interview. * * * Some 80% of people in California live within five miles of a Walgreens store, she said, so the Health Corners could be a valuable tool for reaching people who live in healthcare deserts, or more specifically, Blue Shield network deserts.”
In sad news, the Wall Street Journal reports
Michael F. Neidorff, who as chief executive officer of Centene Corp. transformed a tiny medical insurance firm serving three counties in 1996 into a nationwide giant in government-backed health coverage, died Thursday after what his family described as a long illness. He was 79.
Mr. Neidorff recently took medical leave and had signaled last year a plan to retire in 2022 from the CEO job he held for more than 25 years. Centene announced in March the appointment of Sarah London, who had been vice chairman, to succeed him as CEO.
St. Louis-based Centene is the biggest company in managed Medicaid, contracting with states to provide coverage to people enrolled in the program for lower-income Americans.
Centene offers FEHB HMO coverage through its Health Net subsidiary. RIP.