Midweek Update

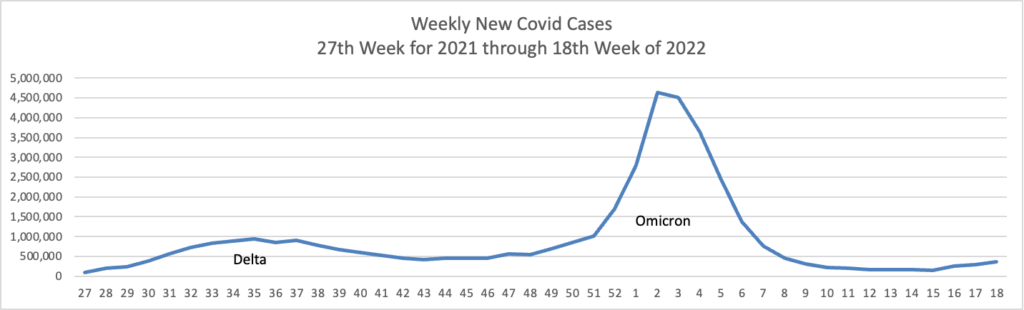
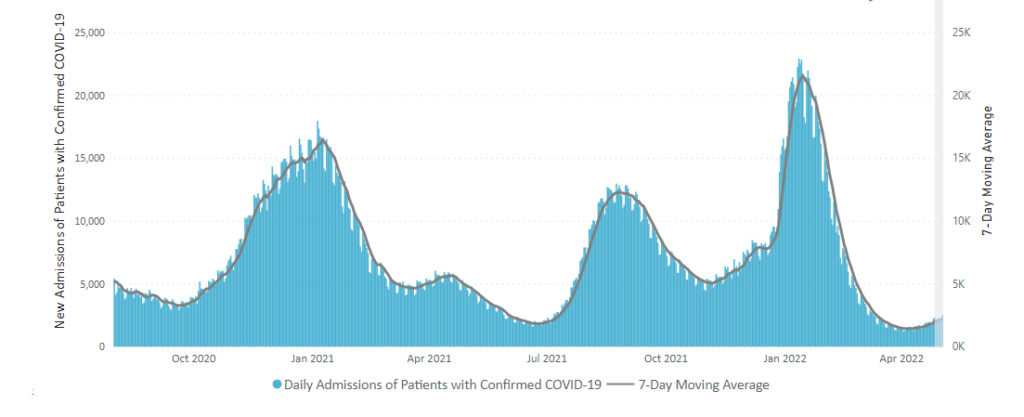
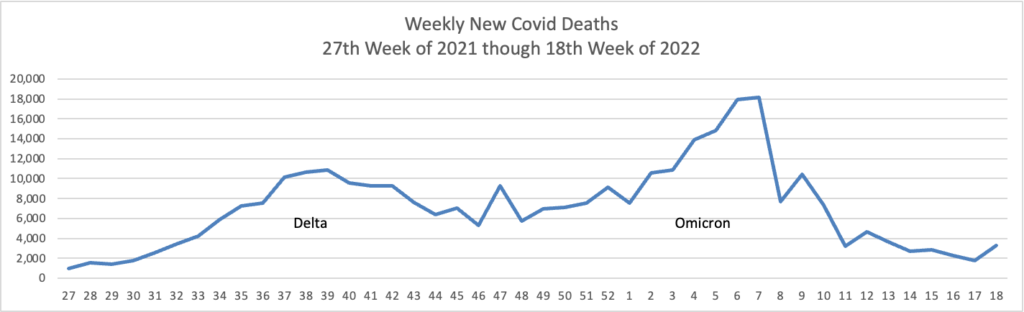
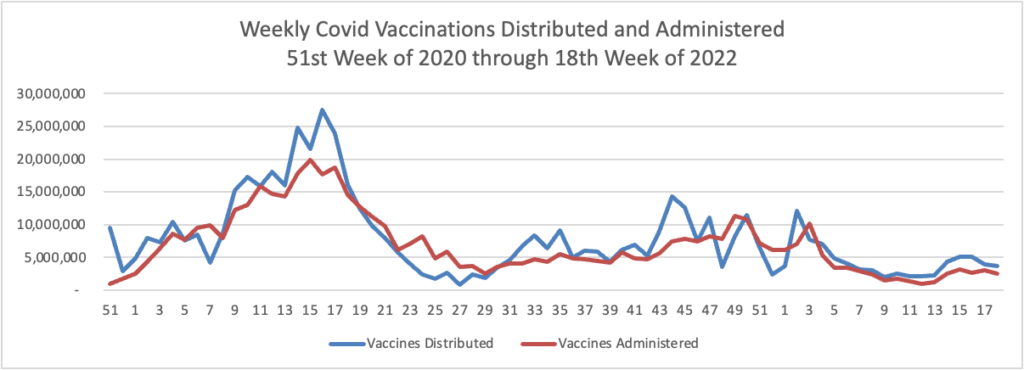
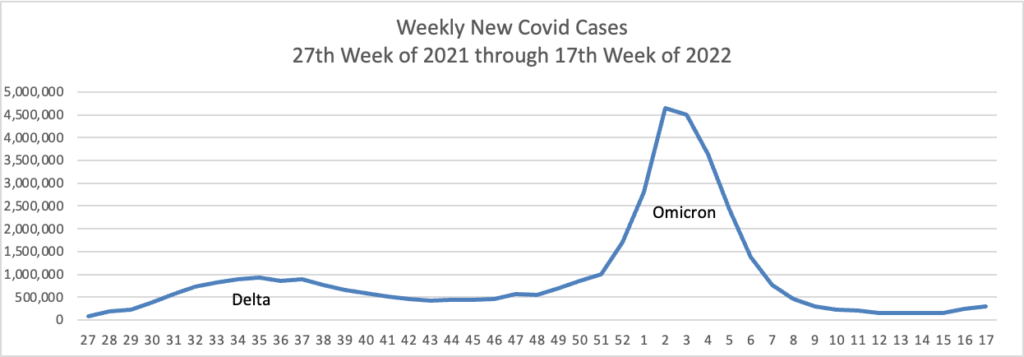
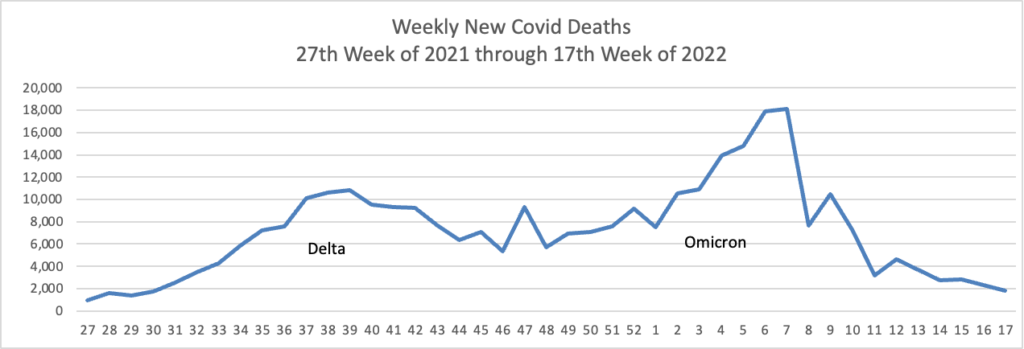
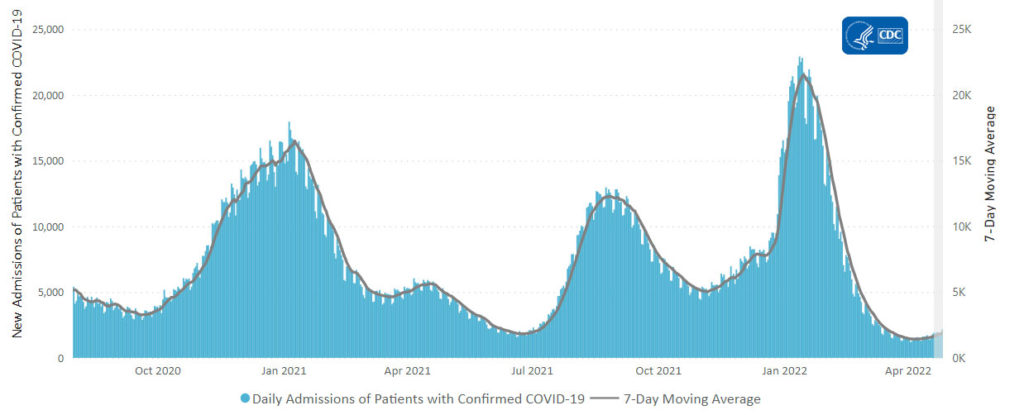
From the Omicron and siblings front —
Health Day informs us
COVID-19 might be easing into a new status as a widely circulating and somewhat harsher version of the common cold, experts say — a virus that folks could contract repeatedly, even if they were recently infected.
“[SARS-CoV-2] is destined to join four of its family members and become an endemic coronavirus that will repeatedly infect individuals throughout their lifetimes,” said Dr. Amesh Adalja, of Johns Hopkins Center for Health Security, referring to the four circulating coronaviruses that cause the common cold.
“It will become one of several respiratory viruses that people contend with, and will become increasingly less disruptive and more manageable with medical countermeasures and the population’s risk acclimatization,” he added.
The FEHBlog recently has pointed out unusual disease cases involving childhood hepatitis , monkeypox, a flu spike, etc. STAT News seeks to put these unusual cases in perspective.
These viruses are not different than they were before, but we are. For one thing, because of Covid restrictions, we have far less recently acquired immunity; as a group, more of us are vulnerable right now. And that increase in susceptibility, experts suggest, means we may experience some … wonkiness as we work toward a new post-pandemic equilibrium with the bugs that infect us. * * *
Marion Koopmans, head of the department of viroscience at Erasmus Medical Center in Rotterdam, the Netherlands, said she believes we may be facing a period when it will difficult to know what to expect from the diseases that we thought we understood.
“I do think that’s possible,” Koopmans said.
This phenomenon, the disruption of normal patterns of infections, may be particularly pronounced for diseases where children play an important role in the dissemination of the bugs, she suggested.
Ruh roh.
From the Rx coverage front, Fierce Healthcare reports
Prime Therapeutics cut per member per month drug costs by 26% in one year through its MedDrive program, which leverages biosimilars to help drive down expenses.
The program uses advanced analytics to flag ways that health plans can cut down drug spend, with a particular focus on the potential of biosimilars. Pharmacy benefit managers are betting on biosimilar products to introduce new competition to popular branded products and drive down costs.
Prime, which serves 33 million members across 23 Blue Cross Blue Shield plans, first launched MedDrive in May 2021 and in its first year the program drove savings by focusing on just three biosimilar categories, the PBM said. Cancer drugs led the way for savings.
The International Foundation of Employee Benefits Plans adds
The U.S. Department of Health and Human Services (HHS) and the Food and Drug Administration (FDA) announced the availability of a final guidance for industry entitled “Importation of Prescription Drugs Final Rule Questions and Answers.” The guidance is intended to help small entities comply with the final rule entitled “Importation of Prescription Drugs.” The final rule was issued to implement a provision of the Federal Food, Drug, and Cosmetic Act (FD&C Act) to allow importation of certain prescription drugs from Canada.
In OPM news, OPM announced a group of new staff appointees at the agency today including a new General Counsel and a new Deputy General Counsel.
From the miscellany department —
- Rebecca G. Baker, Ph.D., the director of the NIH HEAL Initiative, shares insights gained from the Third Annual HEAL Investigators Meeting. HEAL is an NIH branch that focus on creating solutions to the opioid epidemic.
- EHR Intelligence discusses how a National Patient Identifier could boost population health. It is mystifying that Congress has not released funds for this important initiative.
- The Medical Group Management Association identifies four ways medical groups can remove barriers to mammography compliance.
- Health Payer Intelligence outlines 2022 actions today by the U.S. Preventive Services Task Force.
- Fierce Healthcare reports
Despite rising availability in online transparency tools, consumers remain unsure about costs and avoid care as a result, a new survey has found.
The annual consumer sentiment survey was conducted in January 2022 by Healthsparq, a health tech company, and reached more than 1,000 insured Americans. Transparency tools were defined as those provided by payers such as in-network provider search, cost estimates and information on treatment.
The majority (70%) of respondents knew that their health plan offered these, up from 49% last year, and most had used them in the past year. They also said this access helps them better understand their coverage and manage costs. Yet nearly half reported avoiding care due to unclear costs, up from a quarter last year. Care avoidance was even more pronounced among those under the age of 34, at 63%.
Mark Menton, Healthsparq’s general manager, told Fierce Healthcare he suspects that is because the tools exist, but consumers do not know how to access the information.
“I think that’s a hurdle we as an industry need to overcome,” Menton said. “They don’t know where to find this information.”