Tuesday Tidbits

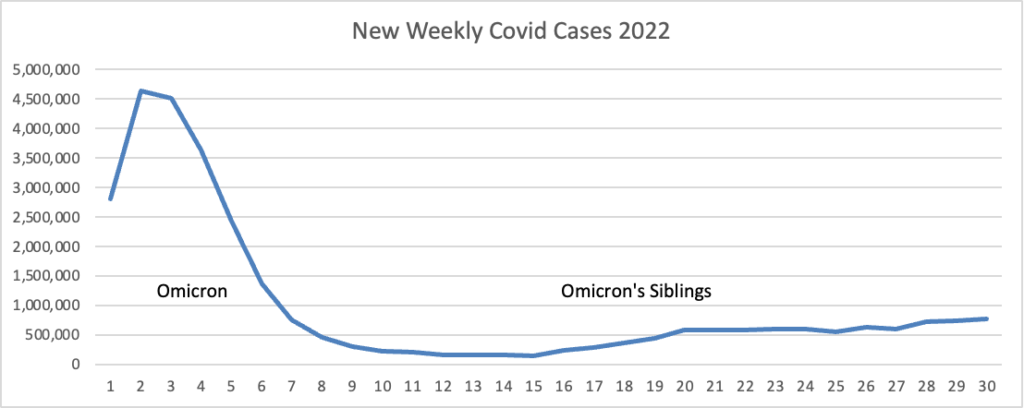
From the Omicron and siblings front, Regulatory Focus reports
[Last week,] both Pfizer and Moderna are seeking FDA authorization for their bivalent COVID-19 vaccines containing components of both the prototype virus and Omicron BA.4/5.
“The FDA is working tirelessly to evaluate the submissions to ensure the data meet FDA’s rigorous standards for safety, effectiveness and manufacturing quality so that these new boosters are available as soon as possible,” Califf tweeted on Thursday, noting that the agency would base its decision on “the totality of available evidence,” including clinical trial data from other bivalent mRNA vaccines, real-world evidence from current vaccines and non-clinical data on the two BA.4/5-containing vaccines.
Califf also said that the agency’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) would not be convened to review the submissions. “FDA will not hold a VRBPAC meeting about these submissions, as the agency feels confident in the extensive discussion that was held in June. VRBPAC voted overwhelmingly to include an omicron component in COVID-19 boosters. FDA has no new questions that warrant committee input,” he wrote.
From the monkeypox front, the Wall Street Journal informs us
A person in Texas who was diagnosed with monkeypox and had a weak immune system has died, Texas state health officials said Tuesday, in what could be the first-known fatality from the virus in the U.S.
The Texas Department of State Health Services said this was the first death of a person diagnosed with monkeypox in Texas. Officials are investigating what role monkeypox played in the death. They said the patient, who was an adult and a resident of Harris County, Texas, was “severely immunocompromised” but didn’t offer additional details.
In a statement, Texas health commissioner Dr. John Hellerstedt said that “monkeypox is a serious disease, particularly for those with weakened immune systems.” He urged those who have been exposed or have symptoms to seek treatment.
Healthcare Dive adds
Concerns over monkeypox vaccine supplies appear to be softening after federal public health agencies initially scrambled to acquire enough doses of the shot.
The Biden administration has been working to boost its supply of vaccines in recent weeks, and so far has made over 1 million vials available to jurisdictions, “which is nearly enough to reach the entire population that’s most at risk,” HHS Secretary Xavier Becerra said during a call with reporters Tuesday.
The HHS also announced on Monday that it will provide about $11 million to support the first U.S.-based productioneffort for manufacturing the Jynneos vaccine at a facility in Grand Rapids, Michigan.
The FDA authorized administering the Jynneos shot intradermally — a method that requires only one-fifth of the usual dose but is just as effective, according to the agency.
However, the vaccine’s developer, Bavarian Nordic, has raised concerns about the method, citing a lack of data and evidence related to its efficacy.
About 75% of jurisdictions that have received the vaccine are administering it intradermally now, Bob Fenton, the White House’s monkeypox response coordinator, said on Tuesday’s call.
From the public health front, CNN Health discloses
CDC Director Dr. Rochelle Walensky has tapped Mary Wakefield — an Obama administration veteran and former nurse — to helm a major revamp of the sprawling agency and its multibillion-dollar budget. Making the changes will require winning over wary career CDC scientists, combative members of Congress, and a general public that in many cases has stopped looking to the agency for guidance.
“If she can’t fix it, she’ll say, ‘It’s not fixable, here’s why, and here’s what needs to be done next,'” said Eileen Sullivan-Marx, dean of the New York University Rory Meyers College of Nursing, who’s known Wakefield professionally for decades.
Also, Specialty Pharmacy Continuum points out
Less than 20% of providers submitted claims using a type of payment code [ICD 10 Z codes] that could help identify and address health disparities that adversely affect patient outcomes, according to a new ICON Market Access report.
The results come amid a growing call for payors and pharmaceutical manufacturers to work together to better address racial and ethnic health inequalities, speakers said during the AMCP 2022 annual meeting.
Such health disparities exist in nearly all U.S. states, said Jessica Cherian, PharmD, RPh, the vice president of content and strategic services for ICON Market Access, citing a 2021 Commonwealth Report, In 2021, her company surveyed 32 payor executives for their perceptions regarding health disparities in racial and ethnic groups, with a targeted focus on medication access and utilization.
Payors typically review data from claims, case manager screenings and more, and use that information to match members to programs that meet their SDOH needs, such as access to care, housing or transportation help, Ms. Fleming said. Payors also track needs through Z codes: additional codes provided in the International Classification of Diseases, Tenth Revision to report nonmedical factors influencing health status. For example, code Z63 would indicate difficulty with a patient’s family/support, such as alcoholism or drug addiction. Approximately 71% of payors in the ICON report used Z codes to monitor SDOH; however, they said less than 20% of submitted claims included these codes.
In payor personnel news, Healthcare Dive informs us
Name: David Brailer
New title: Executive vice president and chief health officer, Cigna
Brailer will assume his new role in early September, and will be Cigna’s first chief health officer.
In his role, he will focus on bringing together Cigna products, technologies and services in new ways in an attempt to drive more value and help improve overall health, according to the release.
He will report to Cigna Chairman and CEO David Cordani and will serve on the company’s enterprise leadership team.
From the healthcare costs front, the HHS Agency for Healthcare Quality and Research released “STATISTICAL BRIEF #543: Trends in Health Insurance at Private Employers, 2008-2021.”
“Highlights
- “Employment-sponsored health insurance at private-sector employers was characterized by increases in premiums and cost-sharing for covered workers in 2021.
- In 2021, average health insurance premiums were $7,380 for single coverage, $14,634 for employee-plus-one coverage, and $21,381 for family coverage, representing increases of 3.2, 3.1 and 3.0 percent, respectively, from their 2020 levels.
- In 2021, the average employee contribution was $1,643 for single coverage, a 7.2 percent increase from the 2020 level. Single premium contributions increased at small (12.3 percent), medium (14.1 percent), and large firms (5.6 percent).
- From 2020 to 2021, average deductible levels for single coverage increased by 3.0 percent to $2,004, and family coverage deductibles increased 3.9 percent to $3,868.
- “From 2020 to 2021, there were no statistically significant changes in enrollment rates or offer rates for small, medium, or large firms.
- “Overall enrollment and offer rates decreased from 2020 to 2021. These decreases are due to an increase in employment among small employers, and a corresponding decrease in the proportion of employees in medium and large firms, which have higher rates for both measures.
- “In 2021, overall eligibility and take-up rates were not significantly different from 2020 levels.”
From the Rx coverage front, HealthDay tells us
Cholesterol-lowering statins are proven lifesavers, but they’ve also gained a reputation for causing muscle aches and pains in a good number of patients.
That reputation is undeserved, according to a new large-scale analysis of data from nearly two dozen clinical trials of statins.
There’s a less than 10% chance that muscle symptoms reported by patients are caused by the statin they are taking, researchers report.
“Our analysis showed that over 90% of muscle symptoms were not attributable to the statin, and those cases that were due to statins occurred mainly within the first year of treatment,” said joint lead researcher Colin Baigent, director of the Medical Research Council Population Health Research Unit at the University of Oxford, in England.
Statins have simply gotten a bad rap when it comes to muscle side effects, Baigent said.
In government contract reporting news, the Society for Human Resource Management reports
The Office of Federal Contract Compliance Programs (OFCCP) has issued a revised directive on compensation compliance, addressing concerns federal contractors had about a previous directive issued earlier this year. Some contractors were concerned that the prior version of the directive intruded upon communications protected by attorney-client privilege.
On the same day the revised directive was issued, OFCCP Director Jenny Yang wrote in a blog post that a top priority for the OFCCP is combating agency pay discrimination.
“Contractors therefore should review the directive and ensure they are engaging in compensation analyses as required by the regulations and be prepared to respond to questions regarding those analyses that are detailed in the directive,” said Guy Brenner, an attorney with Proskauer in Washington, D.C.
“Federal regulations require contractors periodically—or OFCCP interprets now as annually—[to] review their compensation systems to determine whether there are gender, race or ethnicity-based disparities in compensation,” said Sheila Willis, an attorney with Fisher Phillips in Columbia, S.C.