Friday Factoids

Becker’s Hospital Review reports
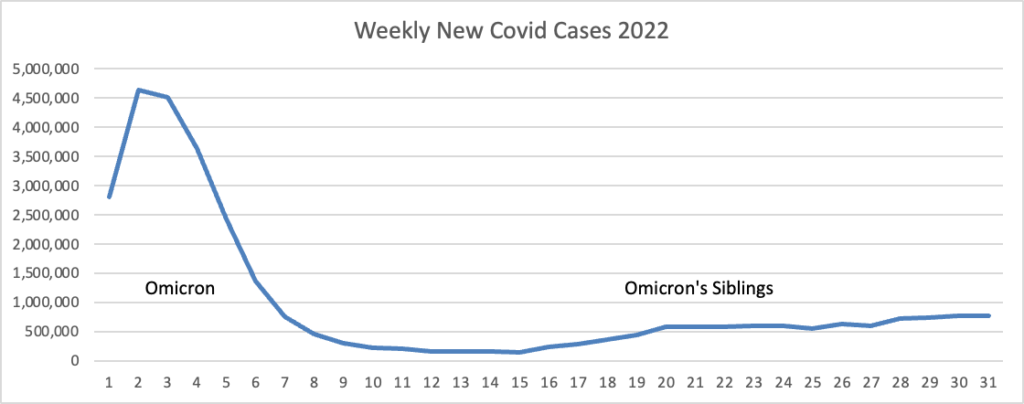
The weekly rate of emergency department visits and hospitalizations for flu, COVID-19 and respiratory syncytial virus peaked in early December, new CDC data shows.
The CDC unveiled two data dashboards Jan. 17 that track emergency department visits and hospitalizations for COVID-19, flu and RSV.
ED visits for flu, RSV and COVID-19 peaked the week ending Dec. 3, hitting a weekly total of 235,850 before falling through December and the first half of January. The nation’s current weekly total was 72,119 as of Jan. 14, according to the ED dashboard. The dashboard uses information from the CDC’s National Syndromic Surveillance Program, which receives data from 73 percent of the nation’s EDs.
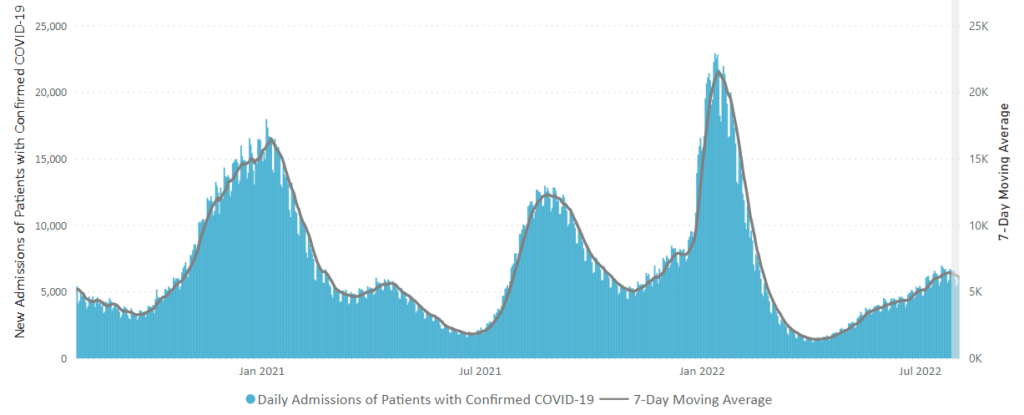
The combined hospitalization rate for flu, RSV and COVID-19 peaked at 22.5 admissions per 100,000 in the week ending Dec. 3. This figure now sits at 9.4 per 100,000 for the week ending Jan. 7, though the CDC said reporting delays may affect the most recent week’s data.
RSV hospitalizations peaked in mid-November, while flu hospitalizations peaked in early December, CDC data shows. COVID-19 admissions also appear to be leveling off nationwide, even as the highly transmissible omicron subvariant XBB.1.5 gains prevalence. This trend suggests the U.S. will see more of a COVID-19 “bump” this winter versus a full-fledged surge, experts told The New York Times.
The CDC’s weekly interpretative review of its Covid stats focuses on these new dashboards this week. The agency’s weekly Fluview report informs us “Seasonal influenza activity continues to decline across the country.”
The Wall Street Journal adds
Three years after health authorities announced the first known Covid-19 case in the U.S., the virus behind the disease remains persistent but thus far hasn’t triggered the severity of the waves seen in prior winters.
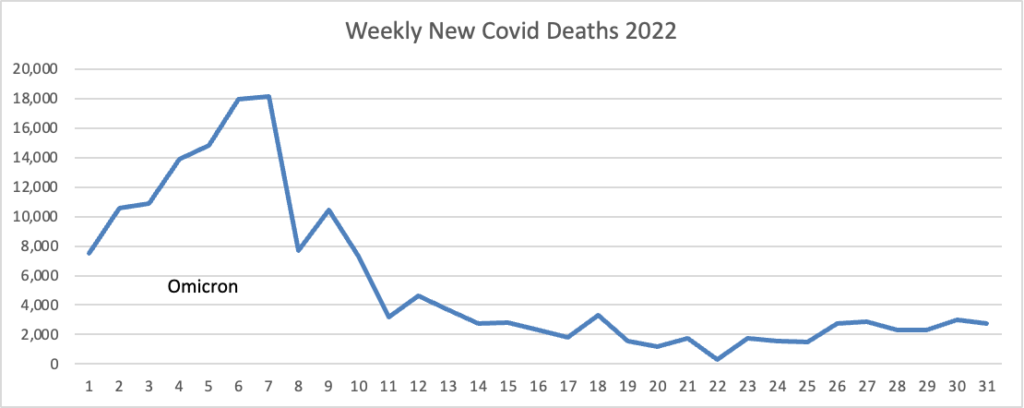
A recent climb in hospitalizations and Covid-19 wastewater readings—two key metrics for spotting trends—appears to have stalled following the quick rise of the Omicron XBB.1.5 subvariant. The U.S. was gripped in significantly more deadly waves at this point in the last two winters, though currently there are still hundreds of deaths reported each day. * * *
At least for now, it appears unlikely new variants are going to cause as substantial illnesses and deaths as the virus did early on and in the prior winter waves, said Jay Varma, a physician and epidemiologist who directs Weill Cornell Medicine’s Center for Pandemic Prevention and Response in New York City. He cautioned that more severe mutations could still emerge. “We seem to have settled into somewhat of a detente with the virus,” he said.
Although the FEHBlog will continue to track Omicron and siblings developments, he has decided to replace Friday Stats and More with Friday Factoids.
Medscape tells us
Public health officials have said for some time that use of Paxlovid, approved under an FDA emergency use authorization (EUA) in December 2021, remains far below the proportion of Americans who could potentially benefit from the therapy.
What’s driving the lackluster uptake remains unknown, so Medscape Medical News took a deeper dive into the challenges surrounding Paxlovid prescribing.
Older Americans remain one of the groups at highest risk for COVID-19 adverse outcomes, including hospitalization and severe illness. However, the survey found that providers also remain reluctant to prescribe Paxlovid in this population for multiple reasons. * * *
The survey found that almost half of patients were on a medication that is contraindicated with Paxlovid and that could not be discontinued (44%). Another finding was that almost the same proportion were on a medication that is contraindicated with Paxlovid, but the risk of discontinuing that medication was too high (41%). Also, the researchers found some patients were on a medication that could interact with Paxlovid, but it was unclear how to manage the interaction (29%).
[Medscape medical editor in chief Dr. Eric Topol said that doctors, in some cases, may be overly concerned about the drug interactions. “There’s a straightforward workaround strategy for nearly all the drug interactions — most commonly statins — which can easily be stopped for 5 days,” he said.
Another concern preventing Paxlovid prescription is renal impairment, the survey reveals. More than one third of respondents, 37%, said they did not prescribe the protease inhibitor combination because of concerns over this condition, which can lower how efficiently medications are cleared by the body.
That’s a helpful study.
In other survey news, MedPage Today informs us
Fewer emergency department (ED) visits end with a prescription for opioids, CDC survey data showed.
The percentage of ED visits with an opioid prescribed at discharge fell from 12.2% in 2017-2018 to 8.1% in 2019-2020, reported Loredana Santo, MD, MPH, and Susan Schappert, MA, of the National Center for Health Statistics in Hyattsville, Maryland, in NCHS Data Briefopens in a new tab or window.
The rate of prescribing at discharge also dropped: in 2019-2020, opioids were prescribed at 36.4 ED visits per 1,000 adults, lower than 50.5 per 1,000 in 2017-2018. The decline was similar for both men and women.
In U.S. healthcare business news —
- Cigna points out the value of integrated health plans a/k/a health plans without carveouts.
A study released today by Cigna (NYSE: CI), a global health service company, finds that triple integration of medical, pharmacy and behavioral benefits resulted in lower health care costs for employers. Conducted by Aon plcThis link will open in a new tab., a leading global professional services firm, the Value of Integration StudyThis link will open in a new tab. [PDF] shows that Cigna’s integrated employer clients saved $148 per member per year in 2021.
Using a similar study method, Cigna then evaluated the financial impact of engaging employees to participate in health improvement programs, such as wellness coaching. The results show even greater client savings for Cigna integrated employer clients, exceeding $1,400 per member per year.
In addition, Cigna found that when individuals with specific high-cost conditions and therapies were enrolled in a triple-integrated health plan and needed specialty medicines, the savings for the health plan were:
- Nearly $9,000 per member per year, increasing to more than $11,000 per member when the specialty drug is for an inflammatory condition like rheumatoid arthritis; and
- Almost $17,500 per year for members who took specialty drugs and have a confirmed depression diagnosis.
- Medpage Today reports, “Switching to [employer-sponsored] high-deductible health plans (HDHPs) spelled trouble when it came to diabetes complications, a retrospective cohort study found.” The report studies health savings account (HSA) – eligible HDHPs versus traditional low-deductible plans. The FEHBlog doesn’t understand why the HSAs don’t balance out the two types of coverage. The article doesn’t compute.
- STAT News relates, “More than a dozen of the country’s large not-for-profit hospital systems descended on this year’s J.P. Morgan Healthcare Conference with a subtle but clear message for bankers and municipal investors: Higher costs in 2022 slowed them down, but they are adamant about increasing revenue by expanding their footprints and hiking prices.” Charming.
- Fierce Healthcare calls our attention to
A new behavioral health solution launched this week aims to make it easier for insurers to connect members with tools that may benefit their mental health care.
Lucet represents the combination of New Directions Behavioral Health and Tridiuum and is a spinout from the Blues network, where its core product cut its teeth. Lucet’s Navigate & Connect platform harnesses a large team of care navigators with an advanced technology stack that allows insurers to better optimize care and access for members.
Shana Hoffman, president and CEO of Lucet, told Fierce Healthcare that the platform enables faster connections to appointments and helps cut through the noise on which solutions a plan may want to bring into the fold.
“What we’re bringing to the market is really an operating system platform for health plans that allows them to reliably connect members to care,” Hoffman said.
- Health Affairs Forefront analyzes “The Fair Price For One-Time Treatments; How Can We Overcome Existing Market Price Distortions?” Check it out.