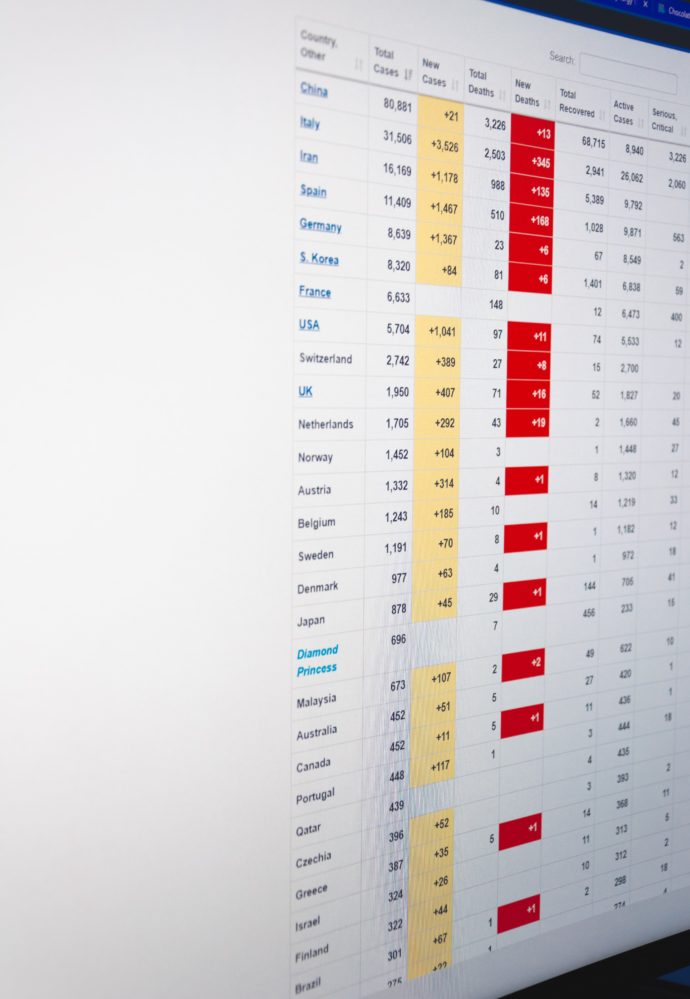
Memorial Day Weekend Update
Congress was scheduled to be out of town this week for State / District work periods. The House however has decided to be in town for votes and a few Committee hearings on Wednesday and Thursday. Congress is considering making some adjustments to the CARES Act’s Payroll Protection Program.
This is a big week for FEHBP carriers because their 2021 benefit and rate proposals are due at OPM by May 31. (Yes, next Sunday.) It is an complicated proposal year because the COVID-19 emergency has disrupted the normal claim payment rhythm.
The Wall Street Journal reports that on a randomized study of hospitalized COVID-19 patients in New York City finding the convalescent plasma (which includes the antibodies created by people who recovered from COVID-19) produced better outcomes. “In the study, the plasma recipients were more likely to remain stable or show improvement in their requirements for supplemental oxygen. They also had improved survival when compared to the control patients.”
The Wall Street Journal also reports tonight about “early signs the U.S. economy is, ever so slowly, creeping back to life.” “If this is the only wave [of coronavirus], it looks like we’ve bottomed out and the normalization process has begun,” said Beth Ann Bovino, U.S. chief economist at S&P Global Ratings.” Much greater testing, improved treatments, and controls on potentially super spreading events hopefully will place our medical system in a position to handle another wave without another great hunkering down.