Friday Items

The FEHBlog apologizes for the fact that his Grammarly program made a hash out of yesterday’s Miscellany post. The FEHBlog discovered and fixed the problems this afternoon if anyone cares to go back to read that post. Lo siento.
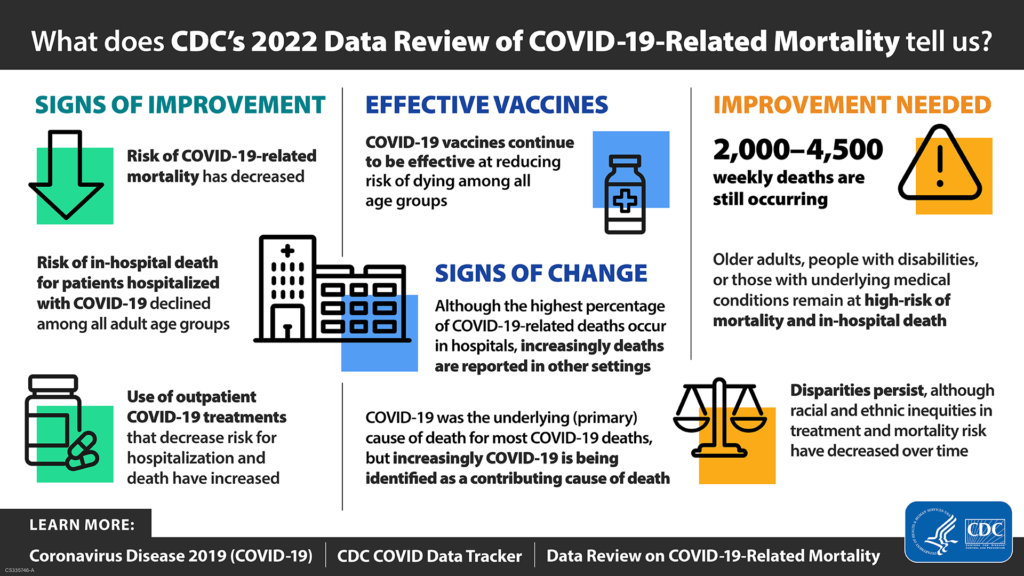
Going forward, the FEHBlog will be posting Covid stats on the first Friday of the month. Here is the CDC’s weekly interpretation of its Covid statistics which focuses on a CDC report on Covid mortality dated November 16, 2022, and summarized below:
The Wall Street Journal adds
Uptake of fall Covid-19 booster shots remains anemic well into November, frustrating public-health experts who blame the lackluster interest on pandemic fatigue and insufficient outreach from officials.
About 31 million people in the U.S. have gotten the updated shots, or roughly 10% of people ages five and older, according to data from the Centers for Disease Control and Prevention. The federal government purchased more than 170 million doses of the new bivalent boosters that target two Omicron subvariants and the original virus strain.
“It has been pretty dismal,” said Rupali Limaye, an associate professor at the Johns Hopkins Bloomberg School of Public Health, who studies vaccine demand and acceptance. * * *
[U]ptake of the modified booster is slow due in part to the limited amount of outreach and type of messaging from health officials, some public-health experts say.
Anecdotally, it sounds like a lot of people are still not aware that the bivalent boosters are available,” said Angela Rasmussen, a virologist at Georgetown University. “If they are, many don’t seem to understand the importance of getting boosted at all—with bivalent or original recipe—and there is a decided lack of urgency in communications about it.”
Some public-health experts say there must be not just more, but also targeted outreach. Celine Gounder, a senior fellow at the Kaiser Family Foundation, said messaging needs to be more targeted at people age 50 and over who are most at risk. Among adults 65 and older, some 27% have gotten an updated booster dose, CDC data show.
“You should be thinking of who is most vulnerable and target the efforts there,” said Dr. Gounder, an infectious-disease specialist and epidemiologist.
The following are the key points from this week’s CDC Fluview:
- Seasonal influenza activity is elevated across the country.
- The majority of influenza viruses detected this season have been influenza A(H3N2) viruses, but the proportion of subtyped influenza A viruses that are A(H1N1) is increasing slightly.
- Two more influenza-associated pediatric deaths were reported this week, for a total of seven pediatric flu deaths reported so far this season.
- CDC estimates that, so far this season, there have been at least 4.4 million illnesses, 38,000 hospitalizations, and 2,100 deaths from flu.
- The cumulative hospitalization rate in the FluSurv-NET system is higher than the rate observed in week 45 during every previous season since 2010-2011.
- The majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this season’s influenza vaccine.
Medscape offers expert opinions on the “perfect storm” of rampant flu and RSV.
In OPM news, the agency posted its Fiscal Year 2022 Performance and Accountability report this week. The Director’s response to the Inspector General’s list of top management challenges is worth a gander. That response begins on page 125.
From the No Surprises Act front, Beckers Payer Issues tells us
The No Surprises Act has prevented millions of surprise medical bills since January, according to new data from AHIP and the Blue Cross Blue Shield Association.
The payer associations gathered their data, published Nov. 17, by surveying 84 health insurance providers representing around 57 percent of the national market.
“Thanks to the No Surprises Act, millions of Americans no longer face a complicated, confusing billing bureaucracy, being harassed by collection agencies, or even potential legal action,” AHIP President Matt Eyles said in a press release.
AHIP and the BCBS Association also surveyed plans for the number of claims submitted to arbitration under the act. They estimated 275,000 claims have been submitted since January 2022, more than the 17,000 claims predicted by government agencies.
AHIP is backing HHS in a lawsuit over surprise billing arbitration from Texas providers, who have support from the largest associations of providers.
The trade association filed an amicus brief in Texas Medical Association v. HHS on Nov. 16.
The lawsuit, filed by the Texas Medical Association in September, challenges the arbitration process established under the No Surprises Act.
The FEHBlog appreciates AHIP’s amicus brief filing. In the FEHBlog’s opinion, the medical association plaintiffs in the Texas case are making a mountain out of a molehill.
A surprising legal development was the Justice Department’s 11th hour notice of appeal filed today in the antitrust challenge to United Healthcare’s acquisition of Change Healthcare which a federal district judge approved last September.
In other Friday items —
Endpoints reports
The emergence of interchangeable biosimilars since the pathway opened up has been slow. But the FDA on Thursday approved the fourth interchangeable biosimilar, which is also the second interchangeable biosimilar insulin product.
Eli Lilly’s Rezvoglar (insulin glargine-aglr), which converted to an interchangeable after an earlier biosimilar approval in December 2021, follows Viatris’ Semglee in seeking out a niche to compete with Sanofi’s blockbuster Lantus (insulin glargine). * * *
These interchangeable designations mean Semglee and Rezvoglar may be substituted at the pharmacy level for Lantus, without a doctor’s prescription, and as long as the state pharmacy law permits the switch.
USA Today reports
Preterm births last year reached their highest peak since 2007 – with more than 383,000 born before 37 weeks of gestational age in the United States, according to a new report.
In 2021, roughly 10.5% of U.S. babies were born premature, according to the annual March of Dimes “Report Card,” which rated the United States at D+. The score dropped from its C- rating in 2020, when the preterm birth rate saw its first decline in six years, a slight decrease to 10.1%.
The report released this week found disparities widened between white mothers and Native and Black mothers, who are already 62% more likely to have a preterm birth and nearly three times as likely as white moms to die of childbirth-related causes. In 2021, Black mothers saw a 3% increase and Native mothers a 6% increase in preterm births, according to the analysis.
Of all groups, Asian and Pacific Islander mothers saw the largest preterm birth increase – an 8% surge – even though births to Asian mothers decreased that year, and they have the lowest preterm birth rate overall.
HHS’s Agency for Healthcare Quality and Research capped off antibiotic awareness week by releasing antibiotic stewardship kits for the acute hospital, long-term care, and ambulatory care settings.






