Weekend update

Congress is back in our Nation’s capitol this week. The House is considering legislative business but is not holding hearings. The Senate is holding hearings and floor votes.
The Wall Street Journal reports
A deeply divided Congress will return to work this week, pushing ahead with partisan priorities in the Senate and House while also gearing up for a fight over how lawmakers will address raising the debt ceiling before a potential default later this year.
The Senate, narrowly controlled by Democrats as it opens its new session, is expected to focus primarily on confirming President Biden’s executive and judicial nominees in the coming weeks. Immigration is emerging as one area of possible compromise after a group led by Sen. Kyrsten Sinema (I., Ariz.) and Sen. John Cornyn (R., Texas) co-hosted a bipartisan delegation of senators to the Texas and Arizona borders during the January recess.
House Republicans, back from a weeklong break, will dive into investigations focused on Mr. Biden, his family and his administration, starting with a hearing on border security early next month that will feature testimony from border patrol agents.
The American Medical Association outlines its wish list for improvements in the Medicare payment system.
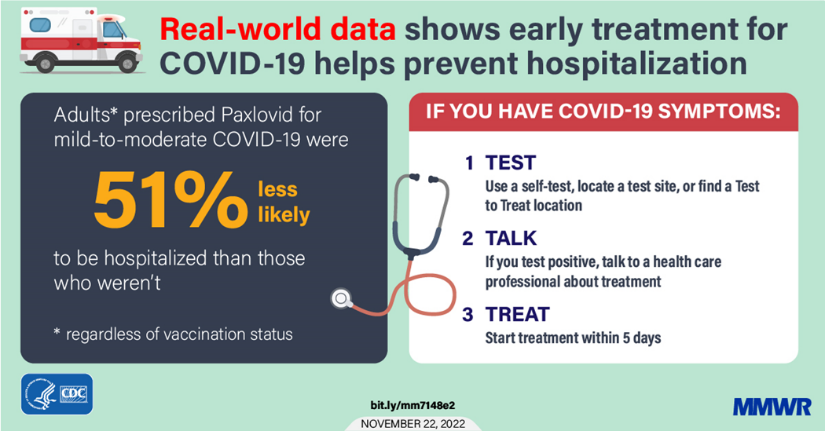
From the Omicron and siblings front
The American Medical Association tells us about what doctors wish their patients knew about Covid reinfections. Oddly the article does not mention the availability of Paxlovid treatment.
Medscape informs folks over age 65 about what they need to know about taking Paxlovid.
The message from infectious disease experts and geriatricians is clear: Seek treatment with antiviral therapy, which remains effective against new covid variants.
The therapy of first choice, experts said, is Paxlovid, an antiviral treatment for people with mild to moderate covid at high risk of becoming seriously ill from the virus. All adults 65 and up fall in that category. If people can’t tolerate the medication — potential complications with other drugs need to be carefully evaluated by a medical provider — two alternatives are available.
The upshot is the older Americans and immunocompromised American should create a treatment plan in consultation with their primary care providers before Omicron shows up at the door.
NPR offers us an update on the state of rapid Covid testing
As the COVID-19 pandemic enters its fourth year, a negative result on a little plastic at-home test feels a bit less comforting than it once did.
Still, you dutifully swab your nostrils before dinner parties, wait 15 minutes for the all-clear and then text the host “negative!” before leaving your KN95 mask at home.
It feels like the right thing to do, right?
The virus has mutated and then mutated again, with the tests offering at least some sense of control as the Greek letters pile up. But some experts caution against putting too much faith in a negative result.
The NPR article provides the details.
In other public health news, Fortune Well reports
A so-called “super strain” of gonorrhea—against which many types of antibiotics are less effective or not effective at all—has been identified in the U.S. for the first time, health officials said Thursday, [January 19] raising further concern that a post-antibiotic era is approaching.
The case, identified in Massachusetts, was successfully treated with ceftriaxone, an antibiotic recommended to treat the disease, state health officials said in a news release. A higher-than-recommended dose wasn’t required to clear the infection, a state public health spokesperson tells Fortune, though the U.S. Centers for Disease Control and Prevention recently doubled the recommended dose.
The newly identified strain showed reduced susceptibility to three types of antibiotics and resistance to an additional three, including penicillin. It marks the first U.S. case in which all recommended drugs were less effective or completely ineffective, the state health department said in a Thursday bulletin to clinicians.
The case serves as “an important reminder that strains of gonorrhea in the U.S. are becoming less responsive to a limited arsenal of antibiotics,” health officials said in a statement.
The U.S. is experiencing “a rising epidemic of sexually transmitted disease,” Dr. Georges Benjamin, executive director of the American Public Health Association, tells Fortune, with some experts referring to the issue as a “hidden epidemic.”
No bueno.
From the mental health care front
- Bloomberg Prognosis calls our attention to a dementia quiz.
Most cases of dementia aren’t linked to lifestyle. But in as many as four in 10 cases, external risk factors — everything from educational level, brain injury and hearing loss to excessive drinking and smoking — may play a role, a report by The Lancet Commission found in 2020. This week, Alzheimer’s Research UK, a charity that funds science and education about dementia, launched an online quiz that draws on that study to help people zero in on what they could change in their own lives to help improve the health of their brains.
“Much of this is about helping people understand that they can be empowered to affect their risk of Alzheimer’s disease,” Paul Matthews, director of the UK Dementia Research Institute at Imperial College London, said in a briefing hosted by the Science Media Centre. “We need to give people the knowledge to make these choices.”
For what it’s worth, The FEHBlog took the quiz which is offered by the British Alzheimers Disease Association. The FEHBlog found it worthwhile.